Table of Contents
ToggleCarboxylic acids are the most common organic compounds with significant acidity. These compounds have a carboxyl group attached to hydrogen (HCOOH), an alkyl group (RCOOH), or an aryl group (ArCOOH). The carboxyl group is made up of the carbonyl group (C=O) and the hydroxyl group (OH).
Structure of Carboxylic acids
The carboxyl carbon and two oxygen atoms in the carboxylic acid group are sp2-hybridized. The three sp2 hybridized orbitals of the carboxyl carbon overlap with the two sp2 orbitals of the two oxygen atoms and the one sp3 hybridized orbital of the alkyl group to form three σ-bonds. The s-orbital of hydrogen and an sp2-hybridized orbital of one of the two oxygen atoms further overlap to form a σ-bonds. The unused p-orbitals (with carbon and each of two oxygen atoms) now overlap sidewise to form the pi-bond, which is partly delocalized between carbon and two oxygen atoms.
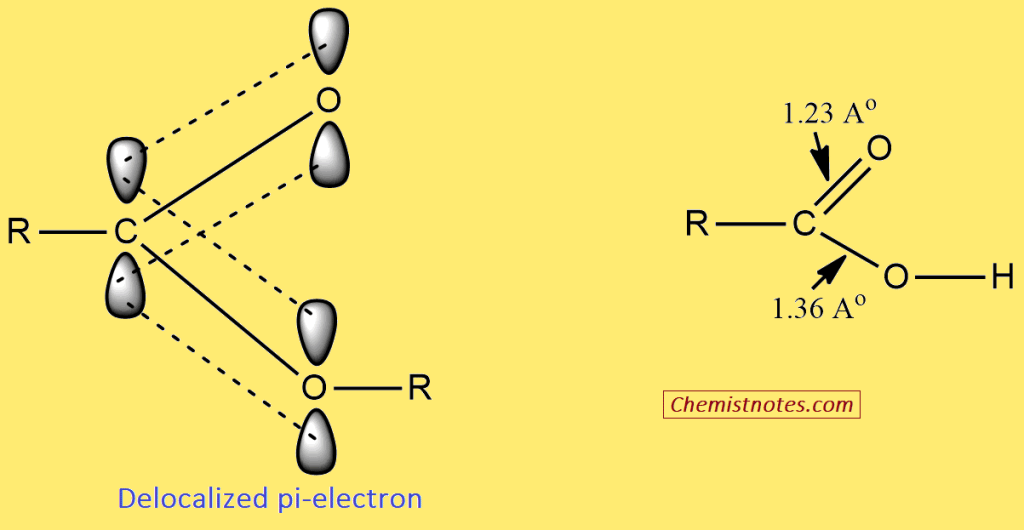
- The C-O single bond of the carboxylic group is shorter (1.36 Å) than the normal C-O single bond in alcohol (1.39 Å).
- The C=O double bond in the carboxylic group is slightly longer (1.23 Å) than the normal C=O bond in a ketone (1.22 Å).
Naming of Carboxylic acids
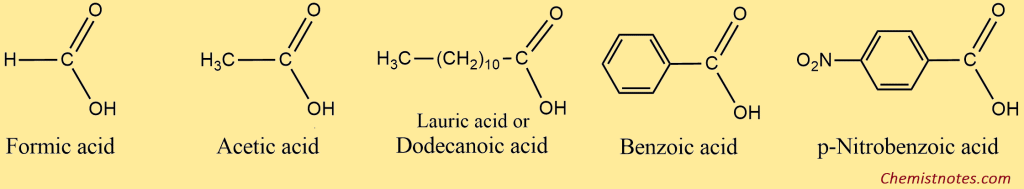
The common names of carboxylic acids are based on their natural sources. For example, methanoic acid is called formic acid because it was first obtained from red ants (Latin: Formica, ant). Acetic acid is so named because it was first obtained from vinegar (Latin: acetum, or vinegar). Similarly, butanoic acid gives off the smell of rancid butter, and hence, it is commonly called butyric acid (Latin: butyrum, butter).
The branched-chain and substituted acids are named derivatives of the straight-chain acids. The position of the substituents is indicated by Greek letters α, β, γ, δ, etc. The carboxyl group bearing carbon atom is assigned the letter α.
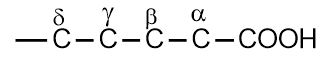
According to the IUPAC system, the longest chain containing the carboxyl group is selected as the parent structure. The name of the acid is then derived from the corresponding alkane by replacing the terminal “e” with “oic acid.” The position of a substituent is indicated by a number.
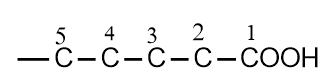
| Molecular Formula | Common Name | IUPAC Name |
| HCOOH | Formic acid | Methanoic acid |
| CH3COOH | Acetic acid | Ethanoic acid |
| CH3CH2COOH | Propionic acid | Propanoic acid |
| CH3(CH2)2COOH | Butyric acid | Butanoic acid |
| CH3(CH2)3COOH | Valeric acid | Pentanoic acid |
| CH3(CH2)4COOH | Caproic acid | Hexanoic acid |
| C6H5CH2CH2COOH | β-phenyl propionic acid | 3-Phenylpropanoic acid |
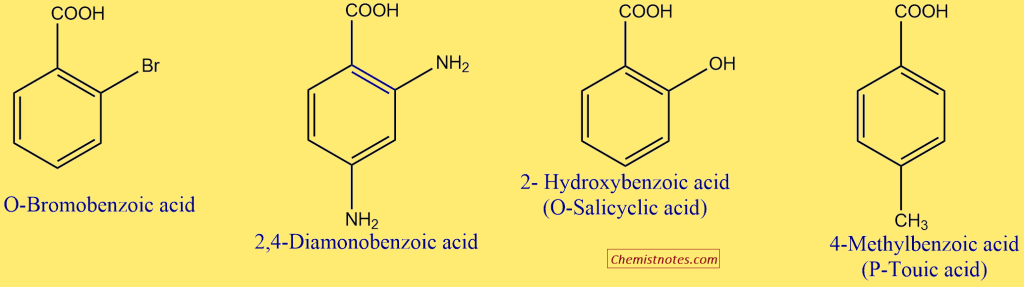
The simplest aromatic acid is benzoic acid, C6H5COOH. Therefore, aromatic acids (Ar-COOH) are usually named as the derivatives of benzoic acid. The methyl benzoic acids are given a special name, i.e., toluic acid.
Physical Properties of Carboxylic acids
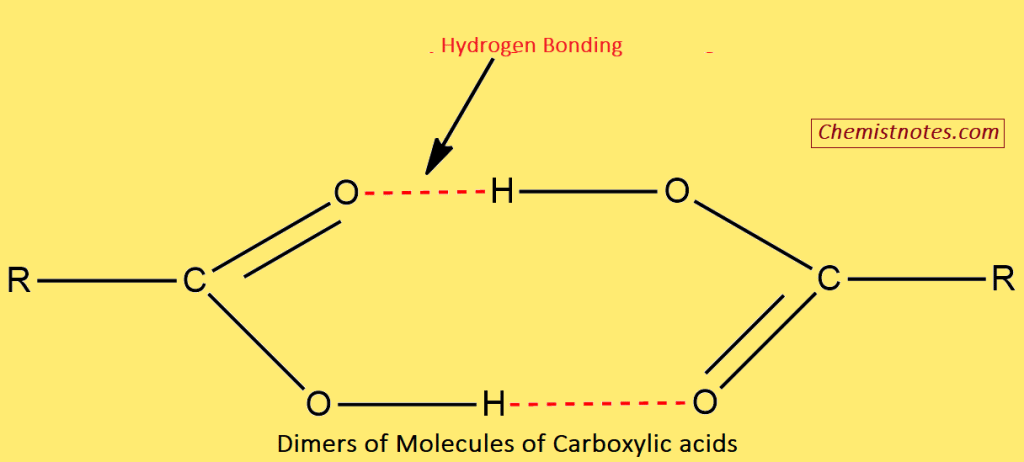
- Carboxylic acids are polar substances, and like alcohol, molecules can form hydrogen bonds with each other and with other kinds of molecules.
- The aliphatic acids show nearly the same solubility behavior as the alcohols.
- The first four carboxylic acids are miscible with water in all proportions.
- The solubility of lower members of a carboxylic acid is due to the hydrogen bonding between the carboxylic acid and water.
- The solubility of carboxylic acid gradually decreases with the increase in molecular mass.
- The parent aromatic acid, benzoic acid, is sparingly soluble in cold water but is soluble in hot water, alcohol, and ether.
- Carboxylic acids are soluble in less polar organic solvents such as ether, benzene, alcohol, etc.
- The carboxylic acid has higher boiling points than the corresponding alcohol of comparable molecular masses. For example, acetic acid boils at 118oC whereas n-propyl alcohol boils at 97oC, where they both have a comparable molecular mass 60.
- The molecules of carboxylic acid are held together by two hydrogen bonds and therefore, they exist as dimers. As a result, the carboxylic acid molecules are held together by strong attractive forces and thus have higher boiling points.