Table of Contents
ToggleBredt’s rule is an empirical rule that tells about the strain in the carbocyclic bridged ring system, especially for the presence of an unsaturated bond. This rule was given by Julius Bredt and thus named Bredt’s rule. Let’s discuss its statement, examples, evidence, applications, and limitations.
Bredt’s rule
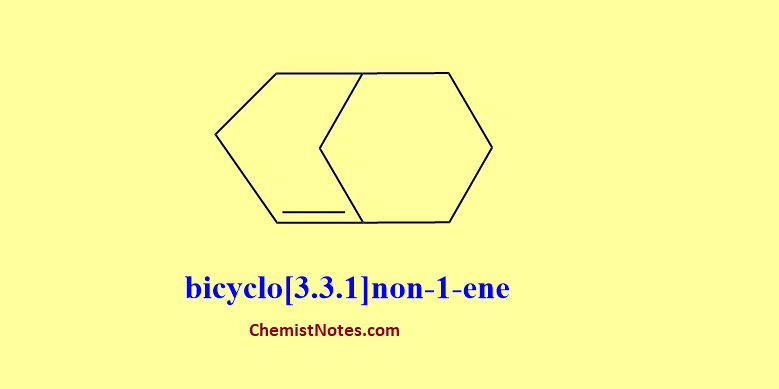
Bredt’s rule states that in a small bridged system, one can not have a double bond at the bridgehead position for reasons of excessive strain. We can take the example of Norbornene or bicyclo[2.2.1]hept-2-ene, which is a very stable compound but its isomer bicyclo[2.2.1]hept-1-ene having a double bond at the bridgehead position is not known.
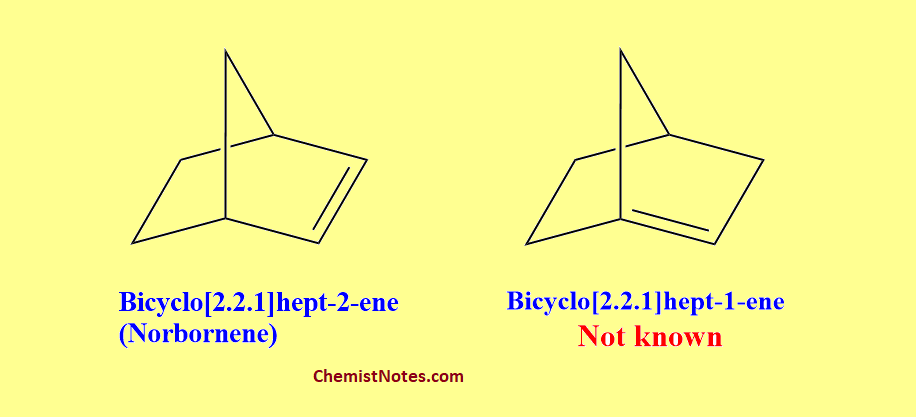
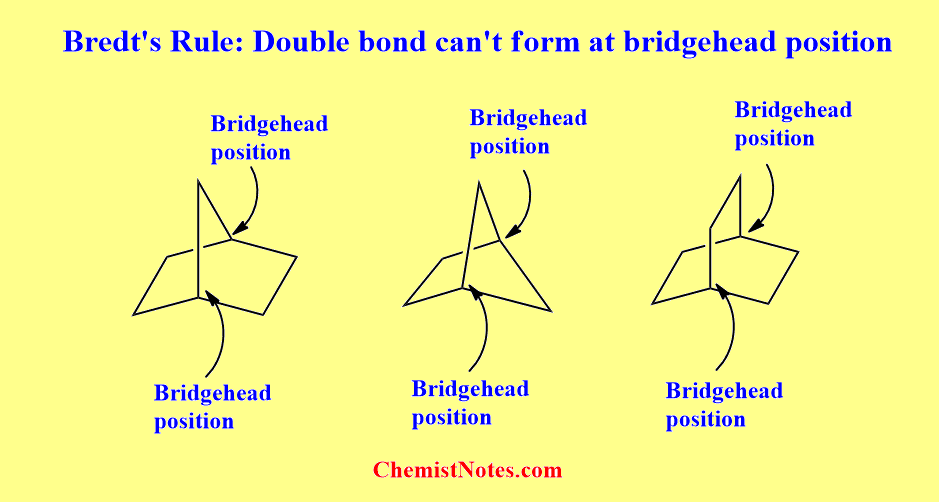
What is bridgehead position or atom? Let’s see the following figure to understand.
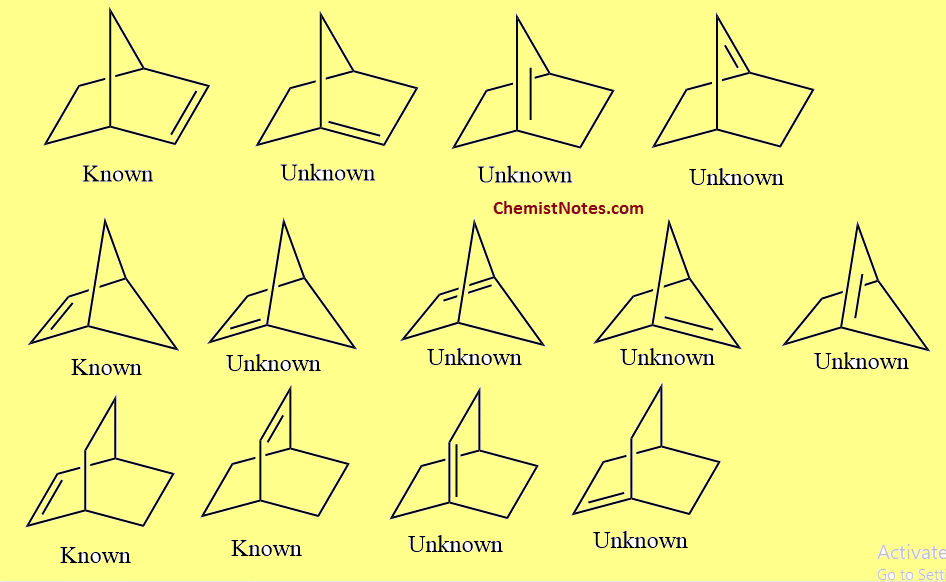
Bredt’s rule examples
Let’s see some examples of this rule.
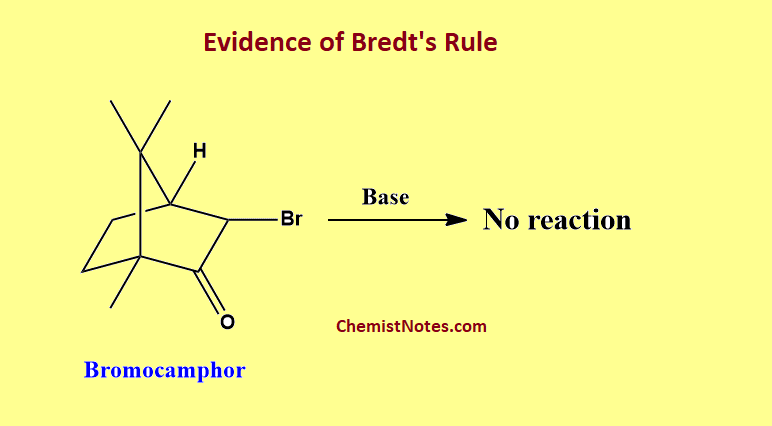
Evidence of Bredt’s rule
When bromocamphor is treated with base, the dehydrohalogenation reaction(elimination reaction) does not occur. To remove hydrogen bromide in this reaction, there must be the formation of a double bond at a bridgehead carbon atom. Therefore, this experiment supports Bredt’s rule.
Similarly, 2-Bromo-norbornane under dehydrobromination reaction gives bicycle[2.2.1]hept-2-ene (norbornene) but not bicyclo[2.2.1]hept-1-ene is not formed in this reaction. All these observations also verify this rule.
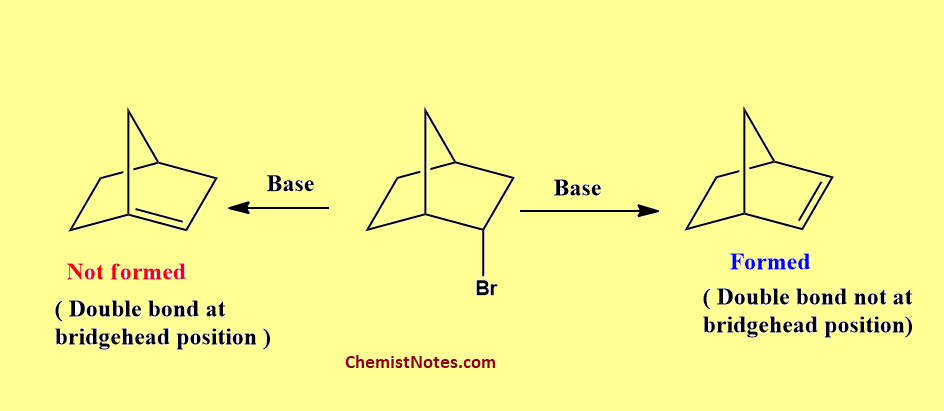
Bredt’s rule explanation
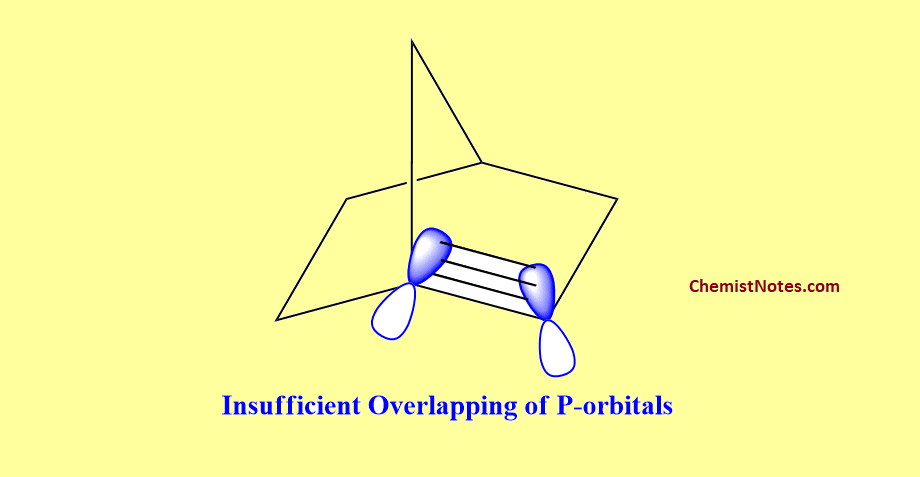
As we know, the position of the double bond in the molecules must be planar. But the planarity required by the double bond is not fulfilled when the double bond tends to form at the bridgehead position. This is because there is no proper overlapping of unhybridized P-orbitals due to inappropriate geometry of the p-orbital of the bridgehead carbon atom.
Bredt’s rule application
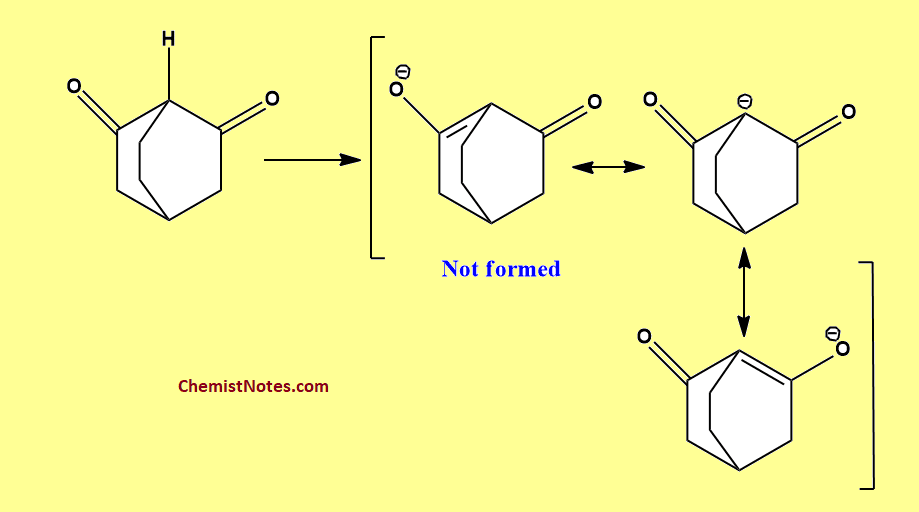
This rule can be used to explain the stability and the acidic property of some bicyclic compounds. For example, the bicycle[2.2.2] octane-2,6-dione does not show acidic properties. It is because according to Bredt’s rule double bond can not form at the bridgehead atoms in such small ring compounds. Hence, the anion formed by the loss of the proton does not acquire stability due to its incapability of resonance.
Bredt’s rule violation
There are some natural products and large rings, the double bonds have been found at the bridgehead position, which may be the fulfillment of the planarity required by the double bond due to puckered structure. Such molecules are known as Anti-Bredt’s molecules.