Table of Contents
ToggleWhat is click chemistry?
A more recent method for creating drug-like compounds called “click chemistry” uses a handful of dependable and practical reactions to accelerate the drug development process.
Natural secondary metabolite-based drug discovery has historically been a labor-intensive, expensive, and time-consuming procedure. The development of leads is still reliant on the dependability of the individual reactions to build the novel molecular framework, despite the development of combinatorial chemistry and high-throughput screening in the last 20 years.
Click chemistry is also called click reaction and has been growing in interest day by day.
Why is click chemistry called a click?
The term “click chemistry” was first used to describe reactions that produced products with high yields and selectivities by the creation of carbon-hetero bonds. The term “click” described the simple connection of two sections of a seat belt buckle for molecular building units.
Invention of click chemistry
Click chemistry was first introduced by Dr. Barry Sharpless in 2001 to explain those reactions which are wide in scope, produce high yields, create only by-products that can be removed without chromatography, and are stereospecific and simple to perform.
One of the interesting things about click chemistry discovery is studied by one literature survey via SciFinder Scholar performed on Dec 31st of 2007 and revealed that a total of 788 publications like books, reviews, articles, and abstracts contain keywords “click chemistry” or “click reaction”.
Click chemistry Nobel prize
The Nobel prize in Chemistry 2022 was awarded to
- Carolyn Bertozzi
- Morten Meldal
- K. Barry Sharpless
“for the development of click chemistry and bioorthogonal chemistry” and the click chemistry in a very interesting way: it is all about one click and the molecules coupled together.
Basic process characteristics
- Simple reaction condition
- The readily available starting material
- The use of no solvent
- Simple product isolation
- minimal purification
- versatile in joining diverse structures without protection step
Classification of Click Chemistry
Click chemistry can be classified into the following types:
- Cycloaddition
- Nucleophilic ring opening
- Non-aldol carbonyl chemistry
- Carbon multiple bond addition
1. Cycloaddition
These contain hetero-Diels-Alder cycloadditions but largely refer to 1,3-dipolar cycloadditions.
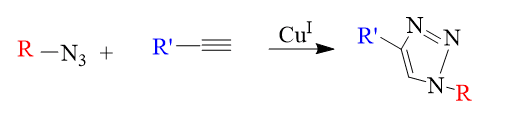
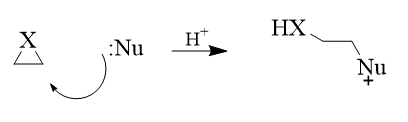
2. Nucleophilic ring opening
These are opening in strained heterocyclic electrophiles such as aziridines, epoxides, cyclic sulfates, aziridinium ions, episulfonium ions, etc.
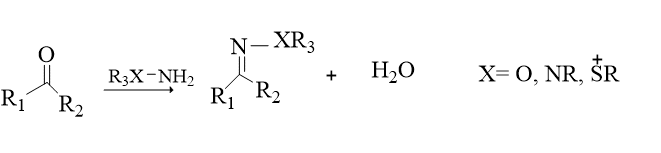
3. Non-aldol carbonyl chemistry
Examples include the synthesis of amides, aromatic heterocycles, ureas, thioureas, hydrazones, oxime ethers, and others. Aldol-type carbonyl reactions often have modest thermodynamic driving forces, thus they take longer to complete and produce side products, disqualifying them from being termed click reactions.
4. Carbonmultiple bond addition
Examples include some Michael additions, epoxidations, aziridinations, dihydroxylations, sulfenyl halide adds, and nitrosyl halide additions.
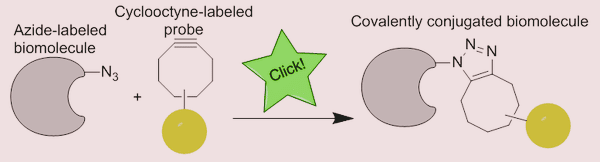
Cycloadditions are the most often employed of the four main classes, especially the CuI-catalyzed Huisgen 1,3-dipolar cycloaddition (HDC) of azides with terminal alkynes to create 1,2,3-triazoles.
Based on the earlier indicated literature search, practically all of the papers cited this click reaction, which has been used in numerous different study fields. The potential of this click reaction and its medicinal applications will be discussed below.
Bioorthogonal Chemistry
Any chemical reaction that can take place inside biological systems without interfering with their natural biochemical processes is referred to as bioorthogonal chemistry.
Purpose of Bioorthogonal chemistry
The idea of the bioorthogonal reaction has made it possible to study biomolecules like glycans, proteins, and lipids in living systems in real time without cellular toxicity ever since it was first proposed.
Why is click chemistry important?
Because of its great chemoselectivity, the click reaction has proven to be particularly effective for modifying functional biomolecules. The copper-catalyzed azide-alkyne cycloaddition click reaction has been used to modify biological oligomers and polymers, including peptides, nucleic acids, and polysaccharides.
Application of Click chemistry
The application of Click chemistry are listed below:
- Click Chemistry in drug discovery
- It is used in biomedical engineering and sciences.
- In pharmaceutical drugs
- nanoparticles modifications
- Natural products modification
Application of Click chemistry in drug discovery
In terms of overcoming the limitations of practical chemical synthesis, increasing throughput, and enhancing the quality of compound libraries, click chemistry reactions are a critical element of the medicinal chemist’s toolkit.
It may be beneficial to combine the diversity-oriented synthesis and ‘privileged’ substructure-based strategy with bioorthogonal reactions using reducing automation and flow systems to increase productivity in order to explore new chemical spaces for drug-like molecules containing a high degree of structural diversity.
The discovery of bioactive chemicals and medicinal agents should benefit greatly from the large compound libraries produced in this manner.
FAQs/MCQs
What is click chemistry?
A type of biocompatible small molecule reaction known as “click chemistry” is frequently utilized in bioconjugation and enables the fusion of desired substrates with certain biomolecules.
Why click chemistry is so-called?
The term “click chemistry” was first used to describe reactions that produced products with high yields and selectivities by the creation of carbon-hetero bonds. The term “click” described the simple connection of two sections of a seat belt buckle for molecular building units.
Who got the Nobel prize for click chemistry?
Carolyn Bertozzi
Morten Meldal
K. Barry Sharpless
What are the types of click chemistry?
The types of click chemistry are
Cycloaddition
Nucleophilic ring opening
Non-Aldol carbonyl reaction
Carbon multiple bond addition
What is bioorthogonal chemistry?
Any chemical reaction that can take place inside of biological systems without interfering with their natural biochemical processes is referred to as bioorthogonal chemistry.
What is the tag for Click Chemistry?
The tag for click Chemistry is it is all about one click and the molecules coupled together.






