Table of Contents
TogglePericyclic reaction mechanisms, definition, examples, and types based on the conservation of orbital symmetry are well discussed below:
The word pericyclic reactions mean around the circle. Pericyclic reactions thus are characterized by a cyclic transition state involving pi-bonds. A pericyclic reaction is a concerted reaction that proceeds through the cyclic transition state. Although most organic reactions take place by way of the ionic or radical intermediate, a number of useful reactions occur in one-step processes as pericyclic reactions that do not form a reactive intermediate.
Such reactions are unaffected by changes in solvent, radical initiators or inhibitors, or any catalysts.
Pericyclic reaction definition
Pericyclic reactions are defined as “the reactions that occur by a concerted cyclic shift of electrons“. This states two main characteristics of pericyclic reactions; (i) the reaction is concerted i.e. bonds are broken and formed simultaneously i.e. at the same time without involving intermediates and (ii) pericyclic reactions involve a cyclic shift of electrons. Besides this, some of the general features of pericyclic reactions are: reversible in nature, mechanism analysis by frontier molecular orbital (FMOs), and forward or backward reactions possessing the same analysis.
Pericyclic reactions require heat (Thermal induction) or UV light (photo induction) and are completely stereospecific. A single stereoisomer of the reactant forms a single stereoisomer of the product. Moreover, two modes of induction (photochemical or thermal) yield products of opposite stereochemistry.
The Woodward-Hoffmann rule which is based on the symmetry of the molecular orbital of the reactants and products, and Hückel-Mobius (H-M) methods are employed for the explanation of pericyclic reactions.
Pericyclic reaction mechanisms
The mechanism of pericyclic reactions can be explained based on the conservation of orbital symmetry proposed by R.B. Woodward and R. Hoffmann. It can be rationalized by the Frontier molecular orbital approach (FMO), aromaticity concept, and correlation diagram.
Based on the FMO approach developed by K. Fukui (1950s), the interactions between the highest occupied orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the reacting species/molecules are considered. The electrons present in the outermost shell of an atom is the HOMO, which can be easily removed with minimum expenditure of energy, while LUMO is the orbital to which electrons removed from HOMO can be transformed into it. Thus, the HOMO of one reactant must interact significantly with the LUMO of another according to the FMOs approach.
Based on the Aromaticity concept, even numbers of antarafacial components in Woodward-Hoffmann notation will cause a pericyclic reaction to proceed through a Hückel transition state, while odd numbers of antarafacial components will cause a pericyclic reaction to proceed through a Möbius transition state. In other words, the involvement of an even number of electron pairs in a pericyclic process will proceed through a Möbius transition state, while an odd number of electron pairs will proceed via the Hückel transition state.
The correlation diagram considers orbital symmetries of the reactants and products. The relative phases of two interacting lobes determine whether the interaction is stabilizing or destabilizing. According to this approach, antisymmetric (A) orbital of the reactant will correlate with (A) orbital of the product, and vice versa for symmetric (S) orbitals of the reactant and products, respectively. However, (A) reactant orbital cannot be correlated with and (S) orbital of the product and vice-versa.
Types of Pericyclic reactions
The pericyclic reaction is categorized into two types:
- Electrocyclic reaction: It is a reversible reaction in which the ring closing or ring opening of a conjugated polyene and a cycloalkene occurs, respectively.
- Cycloaddition reaction: It involves the reaction of diene and dienophile leading to the formation of a ring, where two pi bonds are changed to two sigma bonds.
- Sigmatropic reaction: Reactions in which a group connected by a sigma bond moves to the terminal of a neighboring pi-electron system as part of a coordinated reorganization of electrons are sigmatropic reactions.
- Group transfer reaction: Transfer of one or more groups of atoms from one molecule to another.
Pericyclic reactions example
Some of the examples demonstrating pericyclic reactions are:
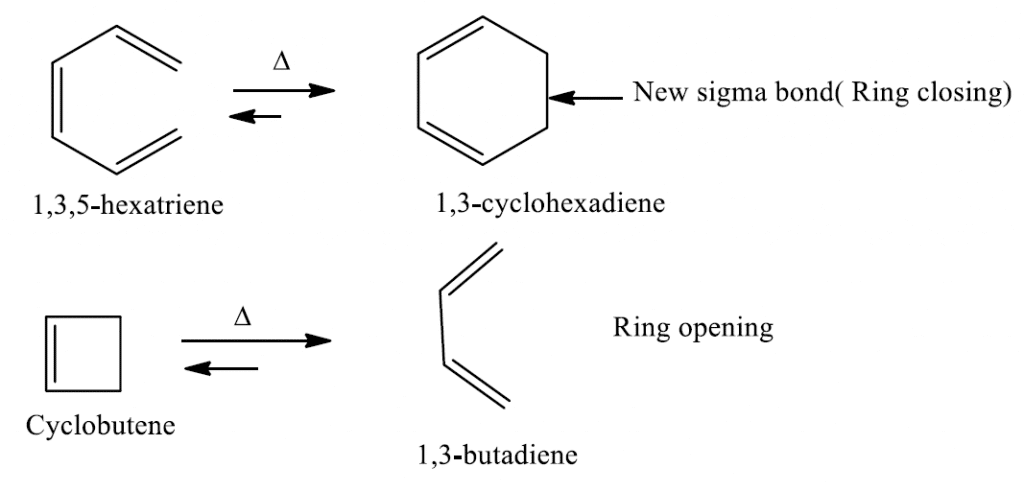
As illustrated in the reaction, 1,3,5-hexatriene undergo ring closure forming 1,3-cyclohexadiene with one more σ bond at the expense of one π bond, while cyclobutene undergoes ring opening and forms 1,3-butadiene with one more π bond in the product at the expense of one less σ bond.
Pericyclic reaction Video
References
- R. B. Woodward and R. Hoffmann, Conservation of Orbital symmetry, Verlag Chemie GmbH, Academic Press, 1971.
- Charles DePuy and Orville L. Chapman, Molecular Reaction and Photochemistry, Prentice-Hall, 1972.
- Peter Skyes, A Guide book to mechanism in organic chemistry, sixth edition.






One Response
I do appreciate your work and help. More over how can I get more of your detailed notes.