Table of Contents
ToggleDiels alder reaction mechanism, examples, stereochemistry, regiochemistry, and application have been discussed here. Diels alder reaction was first of all given by Diels and Alder in 1928
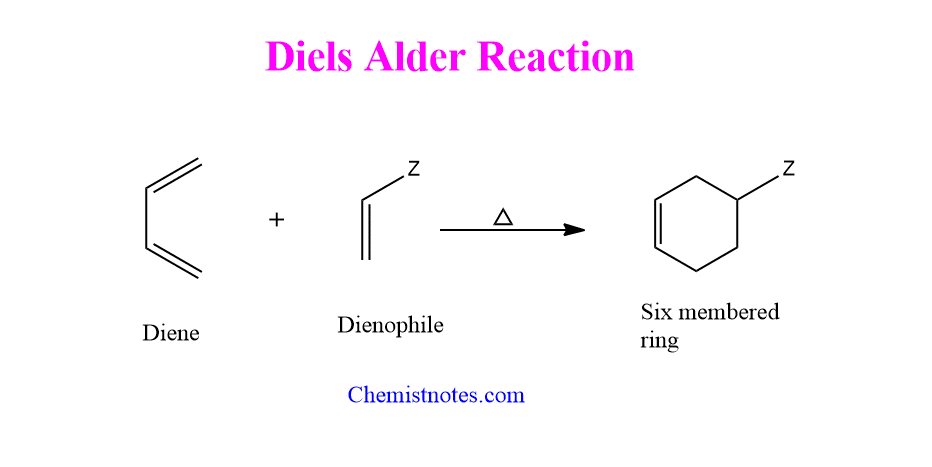
Diels alder reaction
Diels alder reaction definition
The Diels alder reaction is an electrocyclic [4+2]-cycloaddition reaction that produces an unsaturated six-membered ring by combining conjugated dienes with dienophiles such as alkenes or alkynes. This reaction is also known as Diels alder cycloaddition reaction.
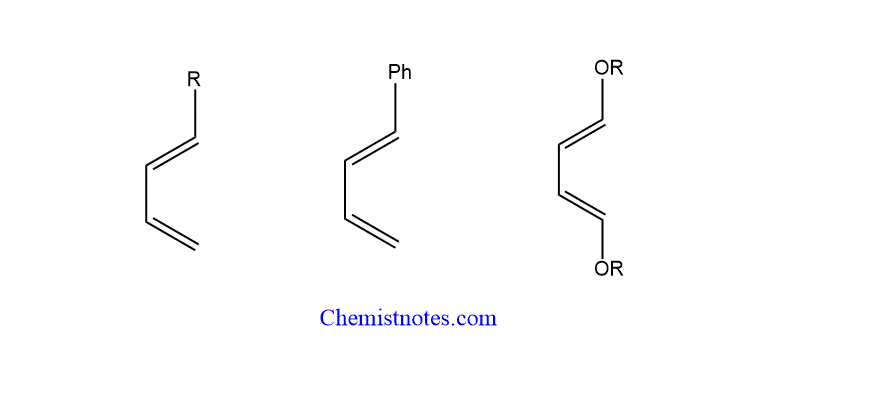
Dienophiles are electron-deficient species, whereas diene is an electron-rich one. As a result, the electron-donating group in the diene favors the reaction, while the electron-withdrawing group on the dinophile speeds up the process
The reactivity of dienes is further enhanced by the presence of electron-releasing groups such as alkyl, phenyl, or alkoxy groups.
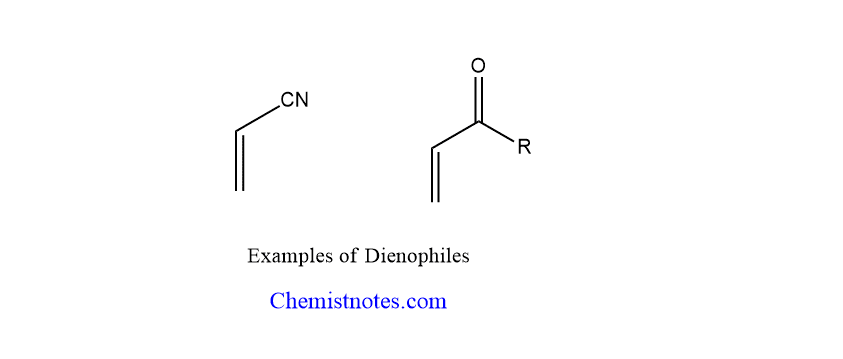
Dinophiles are electron-poor species such as alkenes and alkynes. One or more electron-withdrawing groups shift electron density away from the Pie-bond in a good dinophile. Some examples of dinophiles are conjugated carbonyl compounds, nitro compounds, nitriles, vinyl ethers, etc.
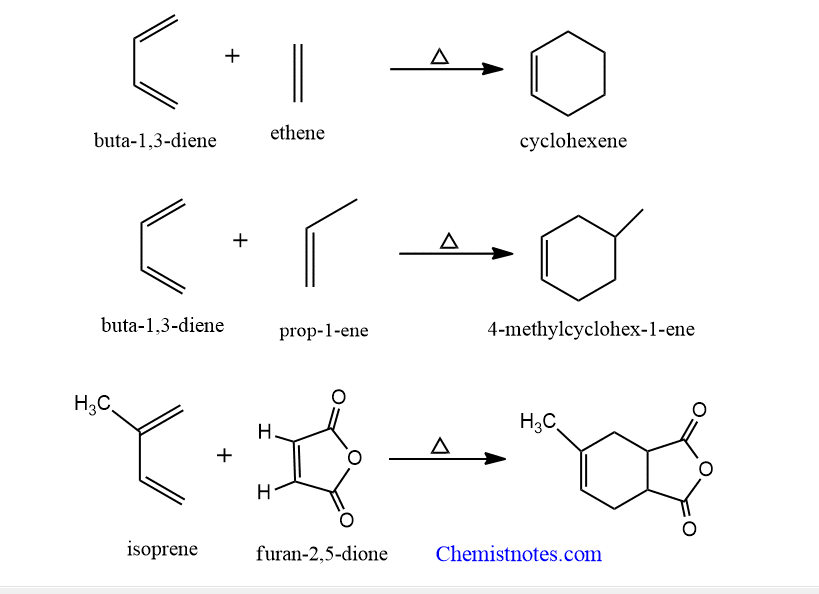
Diels alder reaction examples
Let’s see some examples of diels alder reaction.
Conditions for diels alder reaction
The condition of Diels alder reaction is given below.
- Thermally, the Diels-Alder reaction is permitted, but photochemically, it is restricted. This can be explained on the basis of principle of conservation of orbital symmetry and Woodward- Hofmann rules.
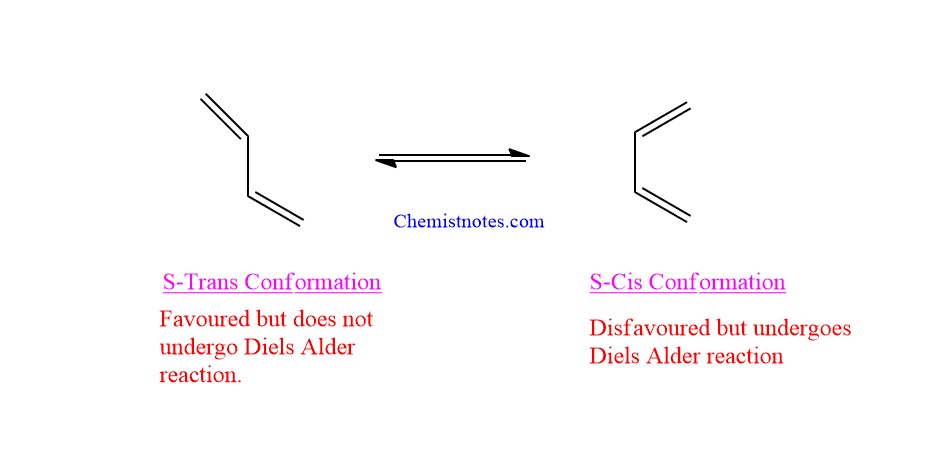
- Another important condition is that the dienes must be in the s-cis conformation because the dinophile cannot reach both ends of the diene at the same time if it is in the s-trans conformation.
For example: Due to steric considerations, cyclobutadiene likes to be in s-trans confirmation, in which two double bonds are separated by a large distance.
Note:
- In the case of cyclic dienes, those that have a steady s-cis conformation are the best for the Diels alder reaction.
- Diels alder reaction is impossible for cyclic dienes that are permanently in the trans conformation.
Diels alder reaction mechanism
Diels-Alder reaction mechanism is a concerted process in which all bond formation and bond breaking take place at the same time.
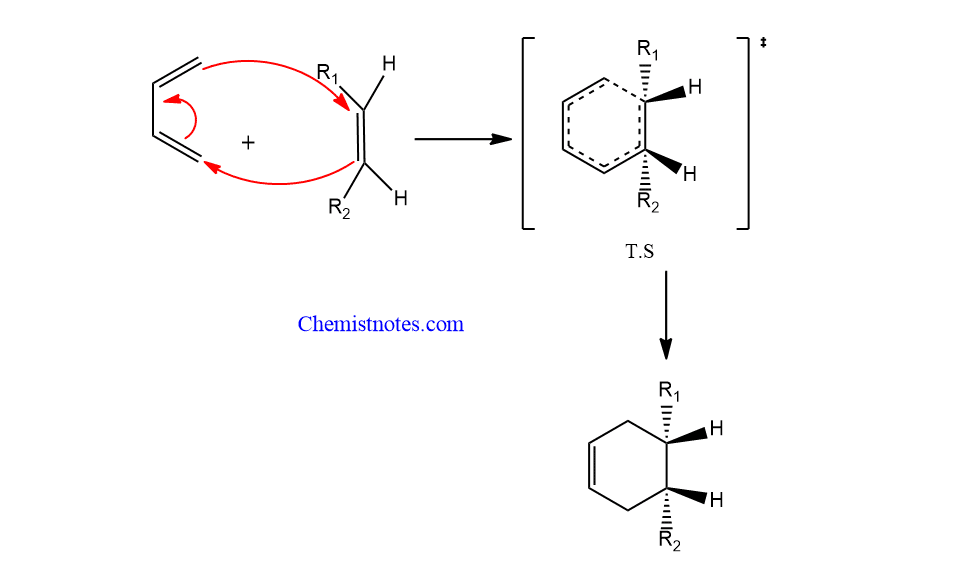
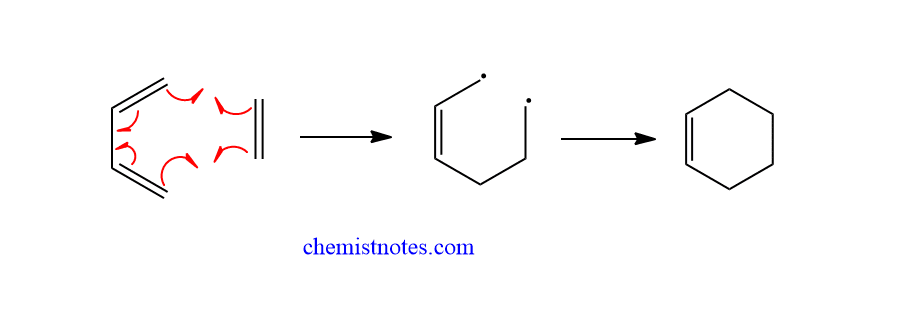
It is believed that the mechanism of diels alder reaction involves the radical mechanism in which diradical intermediate is formed. Let’s see such a mechanism.
Diels alder reaction stereochemistry
During the production of the adduct, the stereochemistry of the diene and dienophile is retained. It means diels alder reaction shows stereospecific with respect to diene as well as dienophiles. Let’s describe the stereochemistry on the basis of dienophile and diene.
A. Stereochemistry of the dienophile
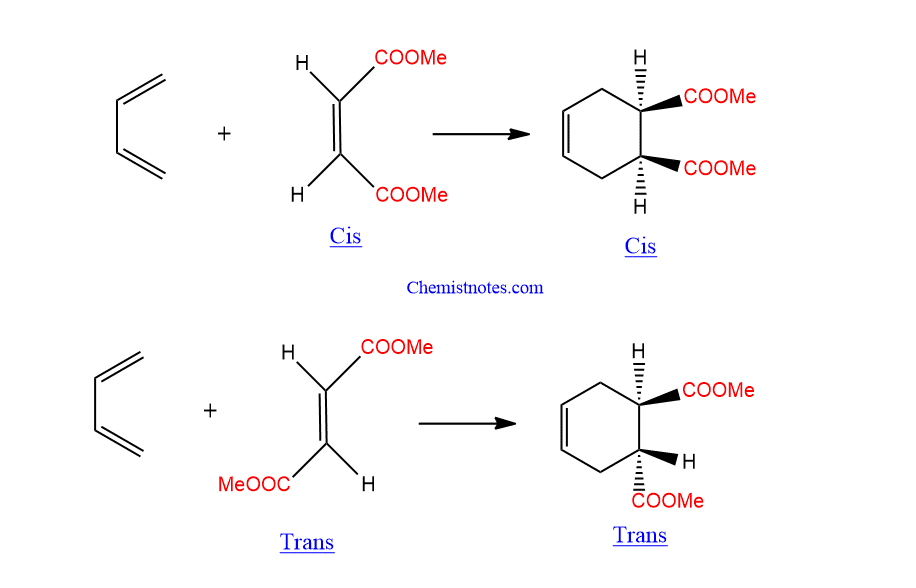
If the dienophile’s substituents are in cis conformation, they will remain in the same cis- conformation in the product. i.e. Cis-orientation of dienophile gives cis-product.
Similarly, In dienophiles, substituents in the trans conformation remain trans in the product.
B. Stereochemistry in diene
The s- cis diene’s terminal vinylic carbon (C-1 and C-4) has two substituents, one of which is known as the outer group and the other as the inner group as shown below in the figure.
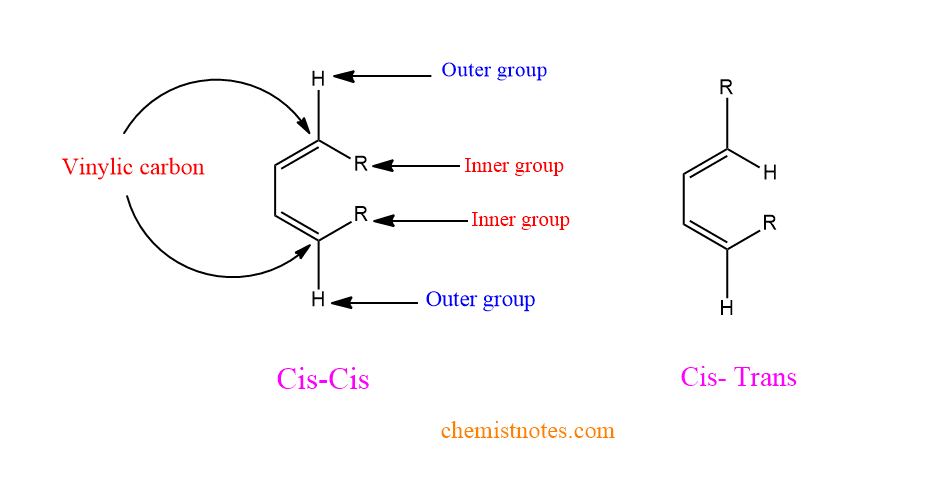
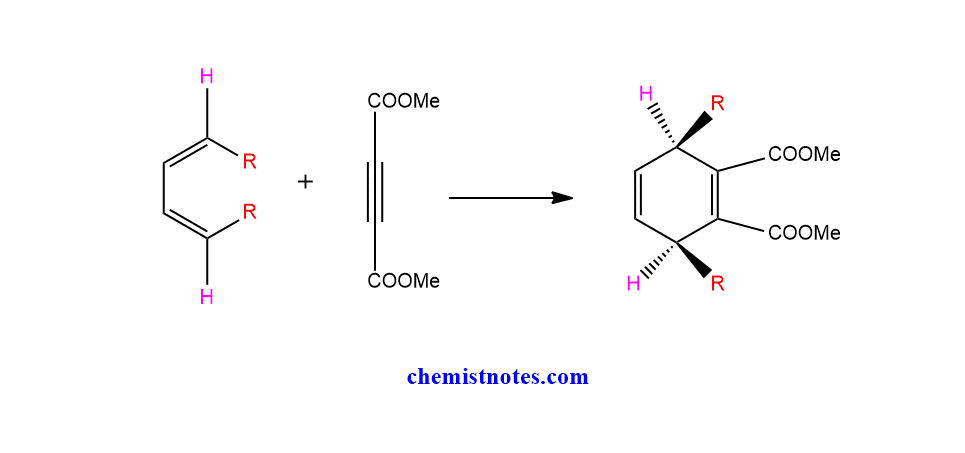
The outside groups are always trans to each other, whereas the two inside groups are cis. The two outer groups will share the same face of the product (six-membered ring), while the two inner groups will share the opposite face.
Let’s see an example.
Endo and Exo stereochemistry:
The Diels alder reaction produces a bicyclic ring structure when the diene is monocyclic. Endo and Exo stereochemistry are required for these ring systems.
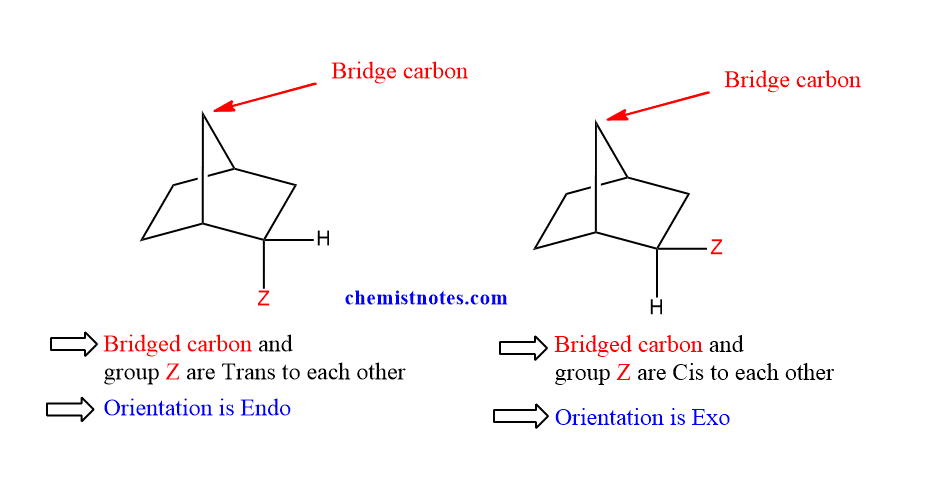
The substituents are trans to the bridge carbon in endo orientation. The substituents are cis to the bridge carbon in Exo orientation. For steric considerations, the exo-product is believed to be more stable than the endo-product. But, Endo products which are kinetically favored, on the other hand, are produced as major products in practice. This is called as endo rule. Let’s see the following examples.
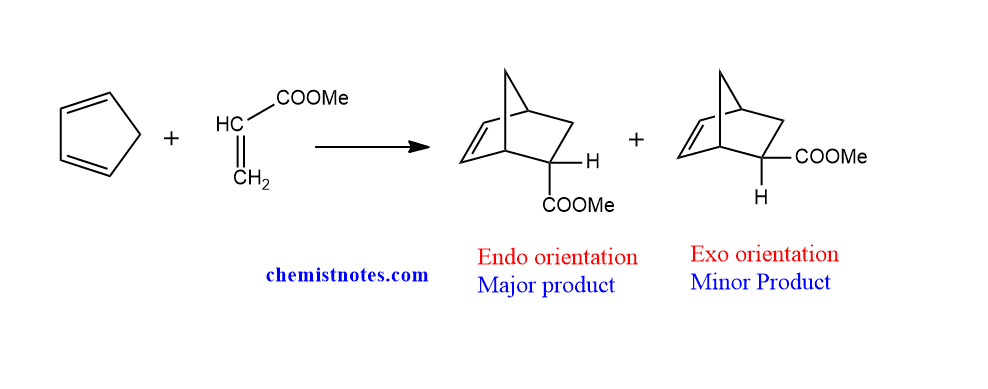
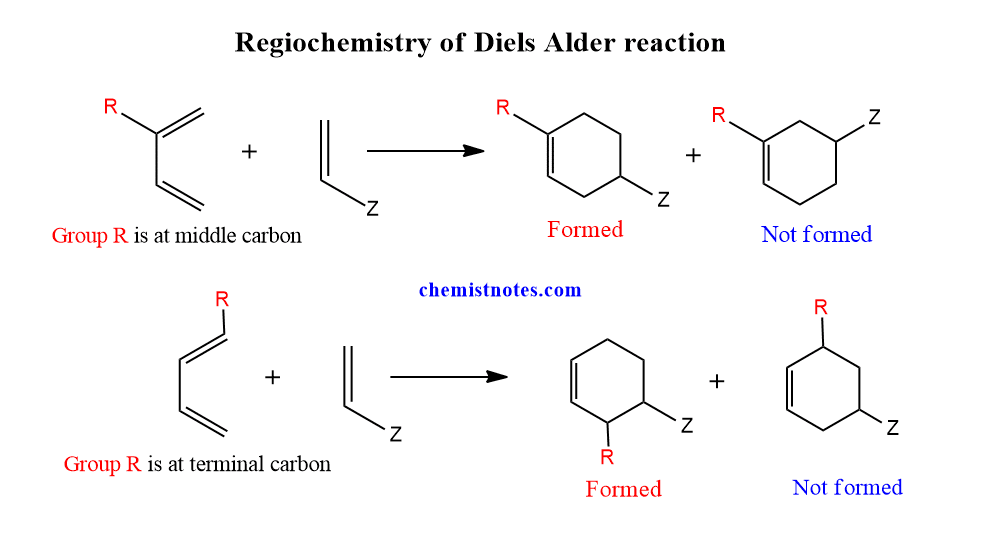
Diels alder reaction regioselectivity
Regiomeric compounds are generated when unsymmetrically substituted dienes and dienophiles react. It means the diels alder product is usually a single isomer rather than a mixture. The following kind of product is generated when an electron-donating group is present on the middle carbon of the diene.
Similarly, when the electron-donating group is present on the terminal carbon of the diene, the following types of products are formed. Therefore, Diels alder reaction shows high regioselectivities.
Industrial applications of the diels alder reaction
The diels alder reaction is mainly used for synthesizing the six-membered ring. Further, it has been reported that its industrial use in the production of pharmacologically active compounds, agrochemicals, cosmetics, and odors.
Diels Alder Reaction Video
References:
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






