Table of Contents
ToggleChloroform is a colorless liquid with the chemical formula CHCl3 and a common organic solvent. This compound is obtained by replacing three hydrogen atoms of an alkane with three halogen atoms. It quickly evaporates into a gas and can harm the eyes, skin, liver, kidneys, and nervous system. Chloroform exposure can potentially lead to cancer.

Method of preparation of chloroform
The industrial method of preparation
From methane
On an industrial scale, chloroform is prepared by chlorination of methane.

From carbon tetrachloride
Chloroform can also be prepared on an industrial scale by partial reduction of carbon tetrachloride with iron filings and steam.

Laboratory method of preparation
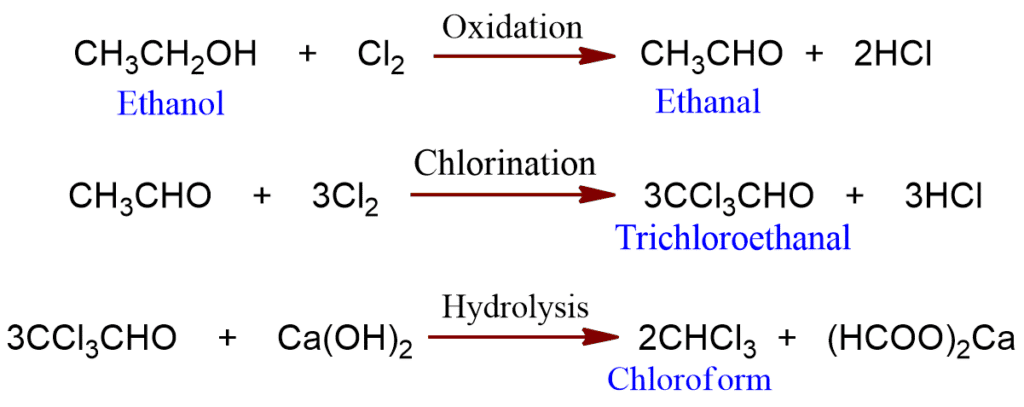
In the laboratory, chloroform is obtained from ethanol or acetone by reaction with bleaching powder and water. In this reaction, bleaching powder serves as a source of chlorine.

The various reactions involved are:
In the case of ethanol
About 50g of bleaching powder is mixed with 100 ml of water to make a paste. This is then transferred into a 500 ml round-bottomed flask. About 15 ml of acetone or 12 ml of ethanol is also added to the flask and the apparatus is arranged as shown in the figure.
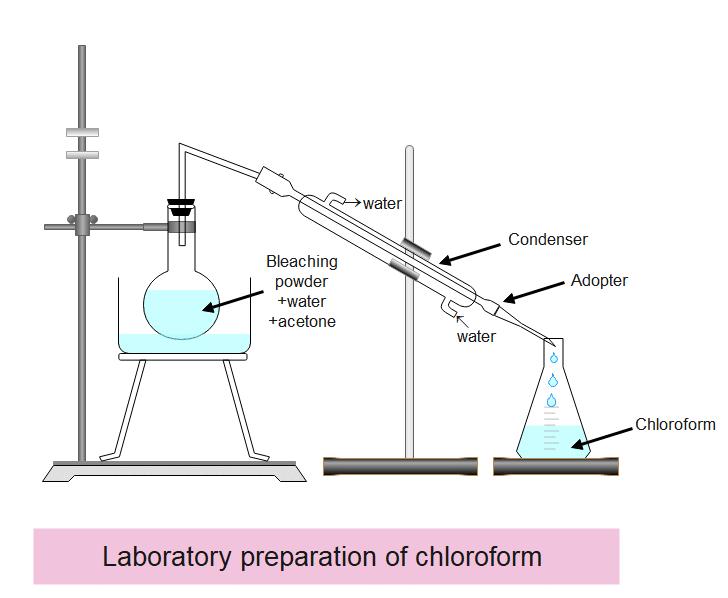
Now the flask is heated gently over a water bath. A mixture of chloroform and water is obtained as distillate. The distillation is stopped when no more chloroform passes over. The mixture of the receiver is passed into the separation funnel and the lower layer of chloroform is separated. It is washed with a dilute solution of sodium hydroxide and then dried over anhydrous calcium chloride. It is finally purified by redistillation and collecting the fraction passing over between 60-65oc.
Properties of chloroform
Physical Properties
- Chloroform is a colorless oily liquid with a sweet smell and a burning taste.
- It is sparingly soluble in water but readily soluble in organic solvents.
- it is heavier than water.
- Its boiling point is 61.2oc and its freezing point is 63oc.
- Its burns with green-edged flame.
- The vapors of chloroform when inhaled cause unconsciousness. Due to this reason, it is used as an anesthetic.
- Chloroform is a good solvent for oils, fats, and waxes.
Chemical Properties
Oxidation
Chloroform is slowly oxidized into a highly poisonous compound phosgene by air in the presence of sunlight.
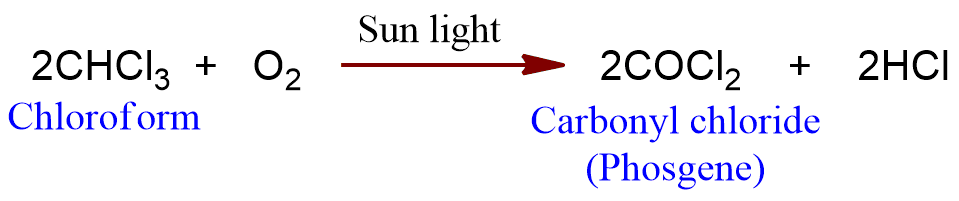
As chloroform is used for anesthetic purposes, therefore, its oxidation to phosgene must be prevented.
Hydrolysis
Chloroform on boiling with aqueous KOH hydrolyzed to form potassium formate.

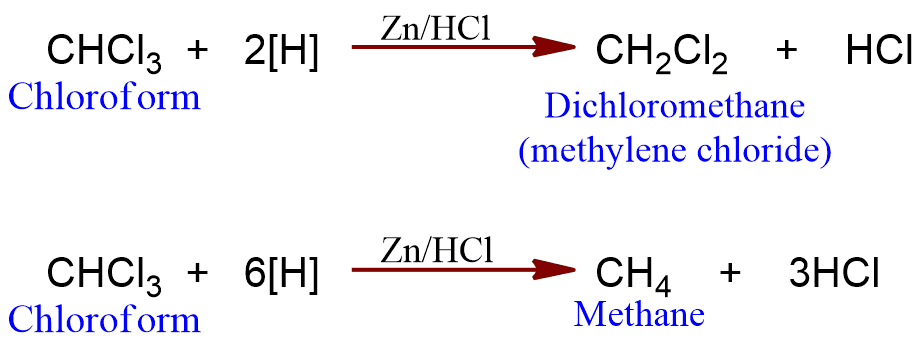
Reduction
Chloroform on reduction with zinc and hydrochloric acid gives dichloromethane. However, on reduction with zinc dust and water, methane is obtained.
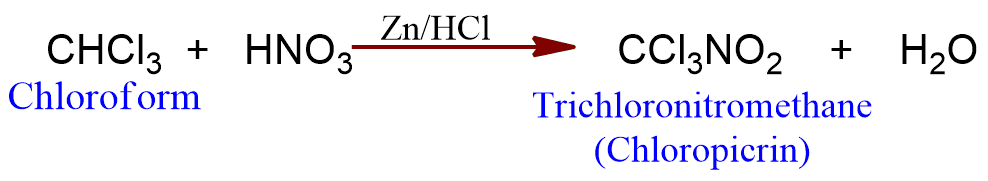
Action with nitric acid
Chloroform on heating with concentrated nitric acid forms chloropicrin which is used as an insecticide and war gas.
Action with Silver powder
Chloroform on warning with silver powder gives acetylene.

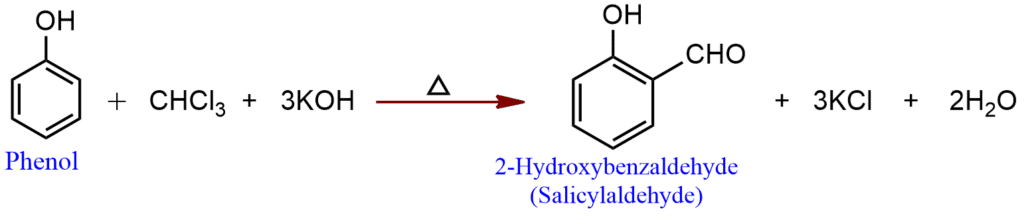
Reimer-Tiemann reaction
Chloroform on heating with phenol and alcoholic potassium or sodium hydroxide solution forms salicylaldehyde. This reaction is called the Reimer-Tiemann reaction.
Uses of chloroform
- It is used as an industrial solvent for fats, waxes, resin, rubber, etc.
- It is used as an anesthetic. But these days it is been replaced by other anesthetics owing to its deleterious action upon hearts.
- It is used as an important laboratory agent.
- It is used for the preparation of important compounds like chloropicrin, chloritone, etc.
- It is also used as medicine.
Related Video
How to make chloroform?
FAQs
What is chloroform?
Chloroform is a colorless liquid with the chemical formula CHCl3 and a common organic solvent.
What is chloroform used for?
It is used as an industrial solvent for fats, waxes, resin, rubber, etc. It is also used as an anesthetic.
References
- Solvents as Reagents in Organic Synthesis (Book by Xiao-Feng Wu). (n.d.). BookAuthority. Retrieved May 10, 2023, from https://bookauthority.org/book/Solvents-as-Reagents-in-Organic-Synthesis/352734196X
- Chloroform | NIOSH | CDC. (2022, December 8). https://www.cdc.gov/niosh/topics/chloroform/default.html






