Table of Contents
ToggleReimer Tiemann Reaction, examples, mechanism, and application, as well as abnormal Reimer Tiemann reaction, have been discussed here. This was first of all given by Reimer and later extended by Reimer and Tiemann in 1876. Reimer Tiemann reaction is also known as Reimer Tiemann aldehyde synthesis or Reimer Tiemann formylation.
Reimer tiemann reaction
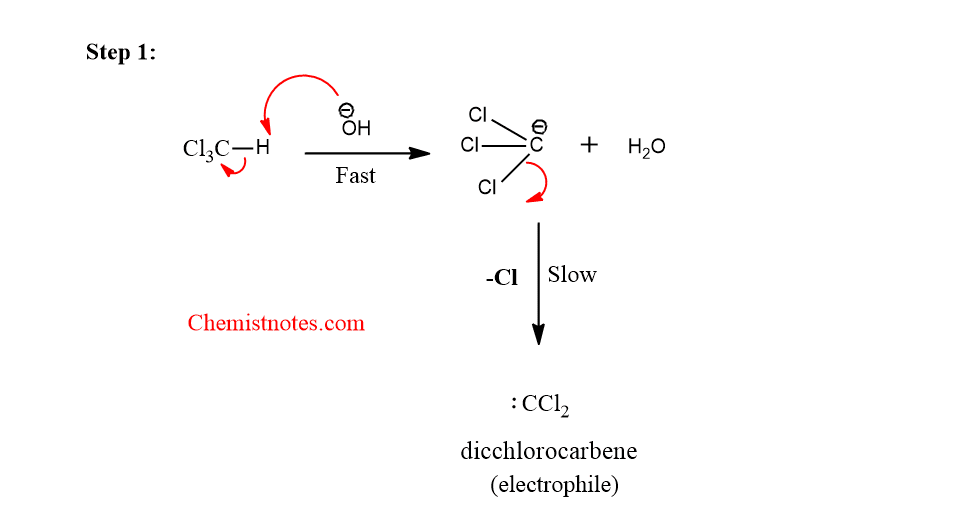
Reimer-Tiemann reaction is an electrophilic substitution reaction in which phenol (other electron-rich aromatic compounds such as naphthols, pyrroles, indoles, etc.) is warmed with chloroform and aqueous alkali to obtain ortho or para- formylated product. Reimer Tiemann reaction intermediate is dichlorocarbene which is generated by the reaction between the chloroform and base.
The limitation of Reimer- Tiemann Reaction are described as:
- Low yields of aromatic aldehyde
- Due to two phase reaction system, mass transfer between two layer can’t occur sufficiently.
Why the ortho-formylated product is favored over the para-product?
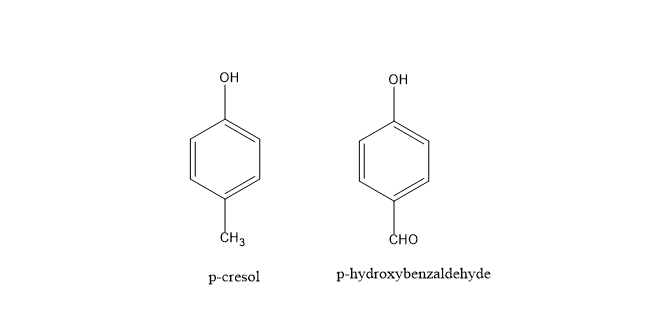
This can be described on the basis of electrostatic effect, solvent, substituents on the aromatic substrate, haloform, alkali base used. For example: In the case of p-cresol, the methyl group donates electrons to the ring and increases the rate of ortho-formylation. But, the rate of para-formylation decreases due to the steric effect of the methyl group. Similarly, in the case of p-hydroxy benzaldehyde, the CHO group is an electron-withdrawing group hence it decreases the rate of ortho- formylation on p-hydroxy benzaldehyde.
Note:
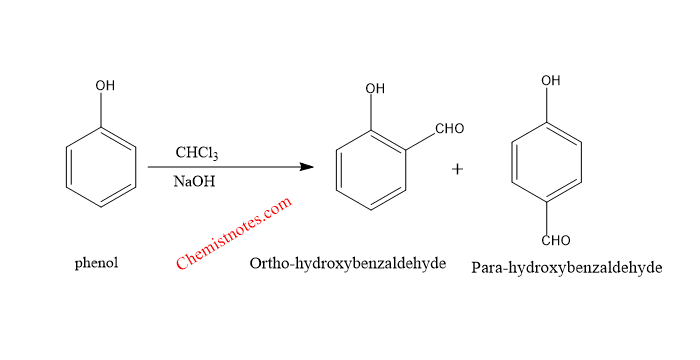
- If phenol is treated with chloroform (CCl3) and base like NaOH, then final product is Salicyaldehyde, also known as 2-hydroxybenzaldehyde.
- If phenol is treated with carbon tetrachloride ( CCl4) and base, then final product is Salicylic acid, also known as 2-hydroxybenzoic acid.
Reimer tiemann reaction example
Reimer Tiemann reaction involves reaction of phenol with chloroform and alkaline solution. The Reimer Tiemann reaction equation is shown below.
Reimer tiemann reaction mechanism
In Reimer Tiemann reaction dichlorocarbene acts as reaction intermediate. This reaction completes in the following steps.
Step 1: Generation of carbene which acts as an electrophile
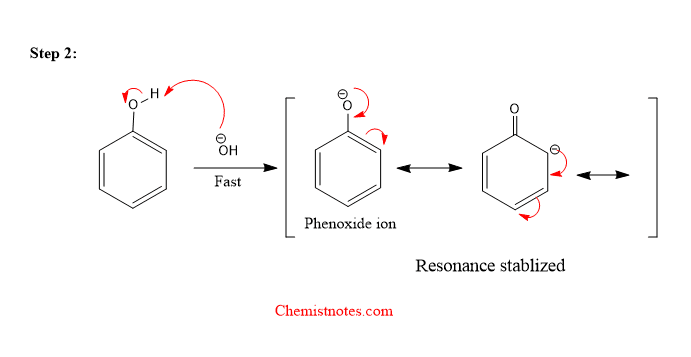
Step 2: Base abstracts the hydrogen atom from -OH group to form phenoxide ion which is resonance stabilized.
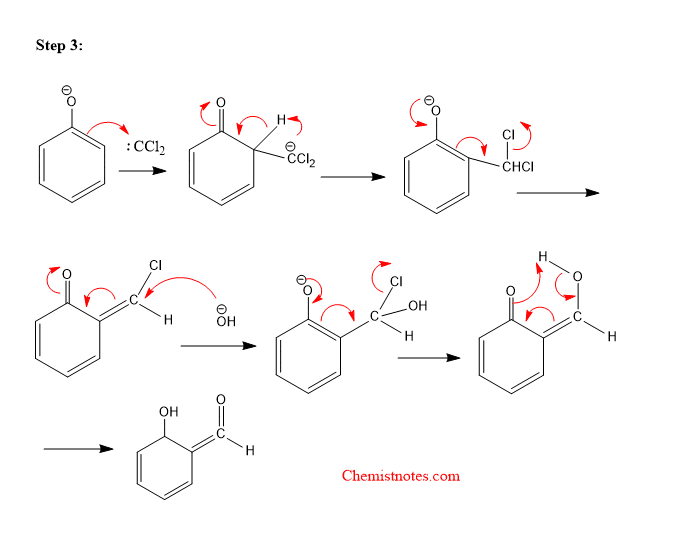
Step 3: Addition of dichlorocarbene to the ring followed by hydrolysis
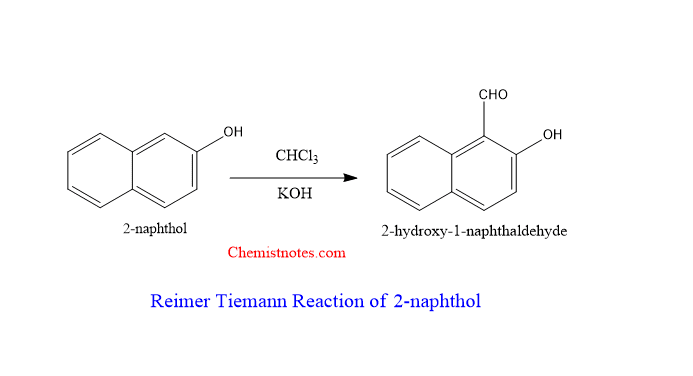
Reimer tiemann reaction of 2-naphthol
A mixture of 2-naphthol is heated with chloroform and an alkaline base, 2-naphthol is converted to 2-hydroxynaphthalene-1-carbaldehyde or 2-hydroxy-1-naphthaldehyde. Let’s see the reaction equation.
Abnormal Reimer Tiemann reaction
Certain molecules such as pyrrole, indene, indole undergo reaction with chloroform and base like NaOH, KOH to give rearrangement product instead of Riemer Tiemann product (o-formylated or p-formylated product). This abnormal type of reaction is known as the abnormal Reimer Tiemann reaction, also known as the Ciamician-Dennstedt reaction. Pyrrole gives 3-halopyridine, indene gives naphthalene and indole gives quinoline when such abnormal Reimer Tiemann reaction takes place. Ring expansion is generally observed during such rearrangement but, is not mandatory. Let’s discuss individual reactions.
Reimer tiemann reaction of pyrrole:
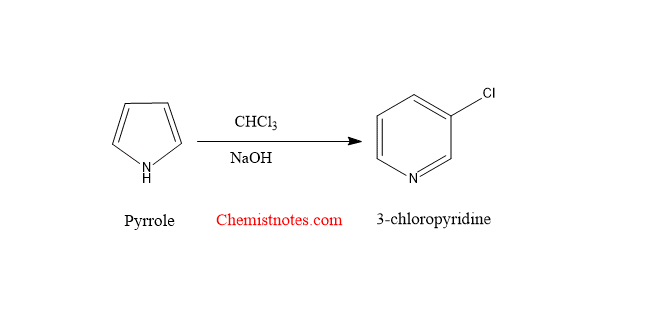
When pyrrole is heated with a mixture of chloroform(CHCl3)and alkaline solution(NaOH), pyrrole is converted to 3-chloropyridine. Pyrrole, when treated with a strong base like NaOH or NaOMe, KOH, dichlorocarbene is formed. The dichlorocarbene is electrophilically combined with pyrrole to form an unstable dichlorocyclopropane, which eventually rearranges to a 3-chloropyridine.
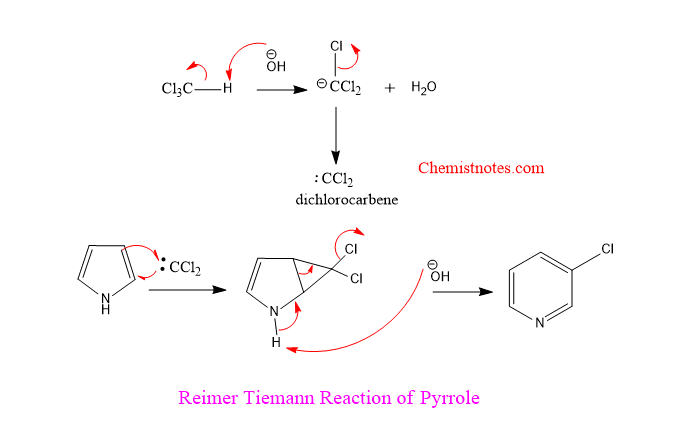
Reimer tiemann reaction of pyrrole mechanism
Let’s see how this reaction takes place.
Step 1: First of all, base abstracts the hydrogen atom of chloroform to generate dichlorocarbene which is an intermediate and acts as an electrophile.
Step 2: The generated dichlorocarbene is inserted into the pyrrole ring to form dichlorocyclopropane which is unstable.
Step 3: The unstable dichlorocyclopropane undergoes rearrangement to give 3-chloropyridine.
The overall process is shown below:
Reimer tiemann reaction of pyrrole mechanism
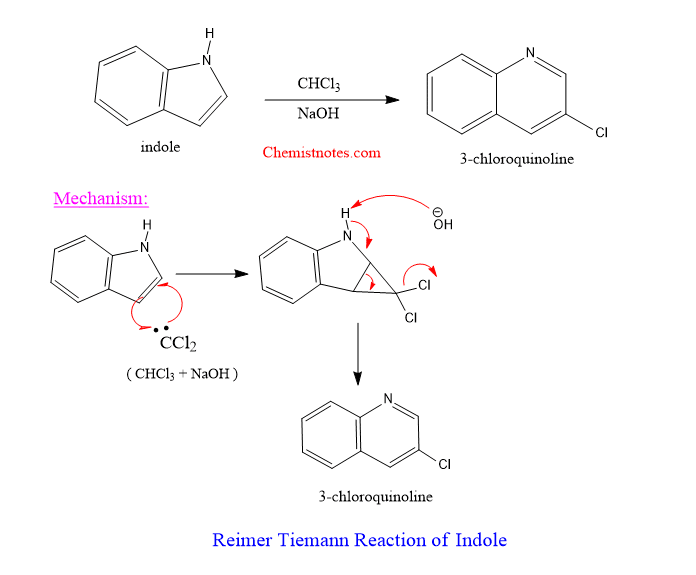
Reimer tiemann reaction of indole
Indole also undergoes abnormal Riemer tiemann reaction to give 3-chloroquinoline as product. The reaction of indole and chloroform in presence of base is shown below with the mechanism.
Application of reimer tiemann reaction:
The uses or application of the Reimer Tiemann reaction is listed below:
- It can be used for the preparation of 3-halo pyridines, naphthalenes, quinolines etc.
- It can be used for the preparation of Salicyaldehyde which is used in making perfumes, as flavor constituent and medicinal purposes. Salicyladehyde is prepared by reacting phenol with chloroform in presence of alkali such NaOH, KOH.
Please comment if you have any problems related to this topic. If you want to learn more about name reactions such as Hell Volhard Zelinsky reaction, click here.
Reimer Tiemann Reaction Video
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






