Table of Contents
ToggleAlkanes are saturated hydrocarbons in which each carbon atom is bonded to another carbon atom and hydrogen atom by a single covalent bond (sigma bond). They are also called paraffin (the Latin word paraffin means little affinity or reactivity). Hence, alkenes are relatively unreactive towards most of the reagents like acids, bases, oxidizing agents, and reducing agents. The alkenes may be divided into the following classes.
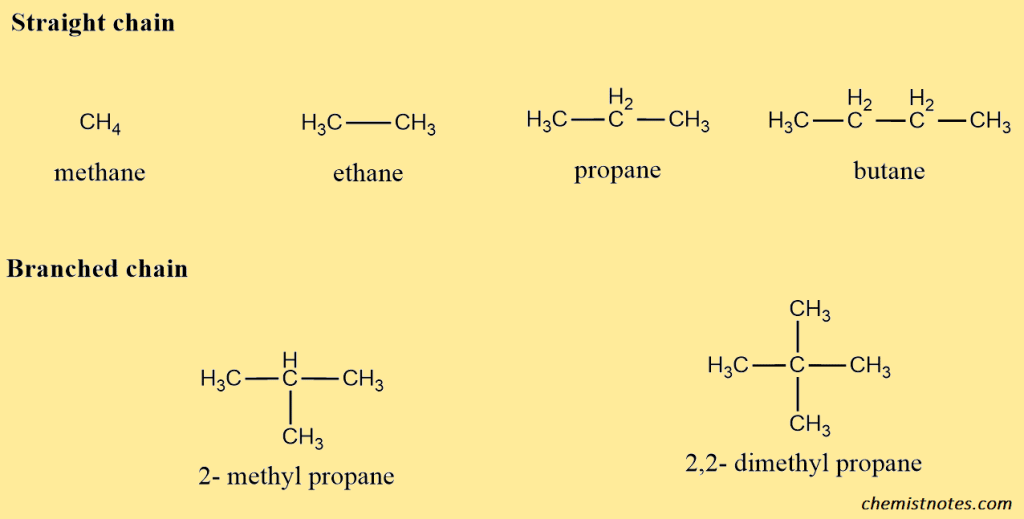
A. Open-chain alkanes: Open-chain alkanes are simple alkanes made up of carbon and hydrogen only and consisting of a long open carbon chain. They are represented by the general formula CnH2n+2, where n is the number of carbon atoms in the molecule. Open-chain alkanes may be straight-chain, or branched-chain compounds. for example
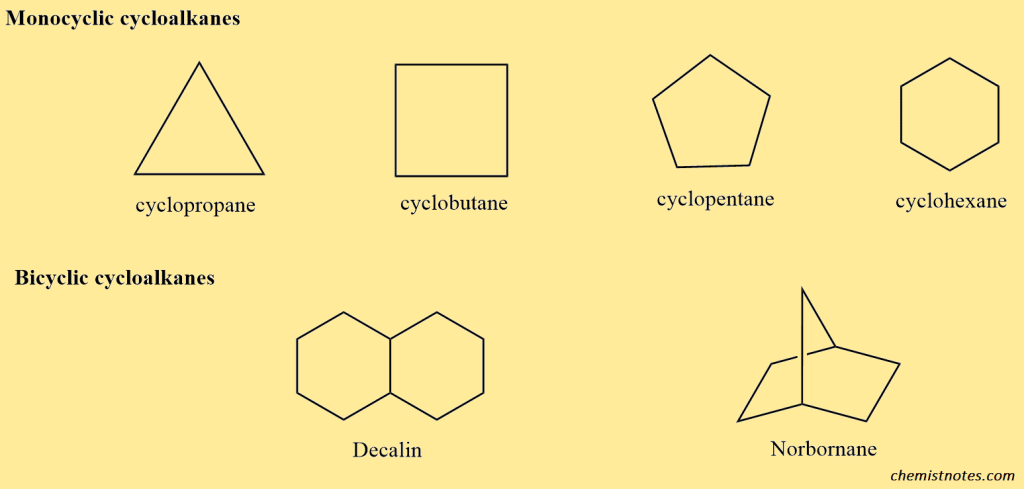
B. Cycloalkane: Cyclic aliphatic compound alkanes are known as cycloalkanes, which are represented by the general formula CnH2n (monocyclic) or CnH2n+2 (bicyclic). Some cycloalkanes are given below.
Structure of alkanes
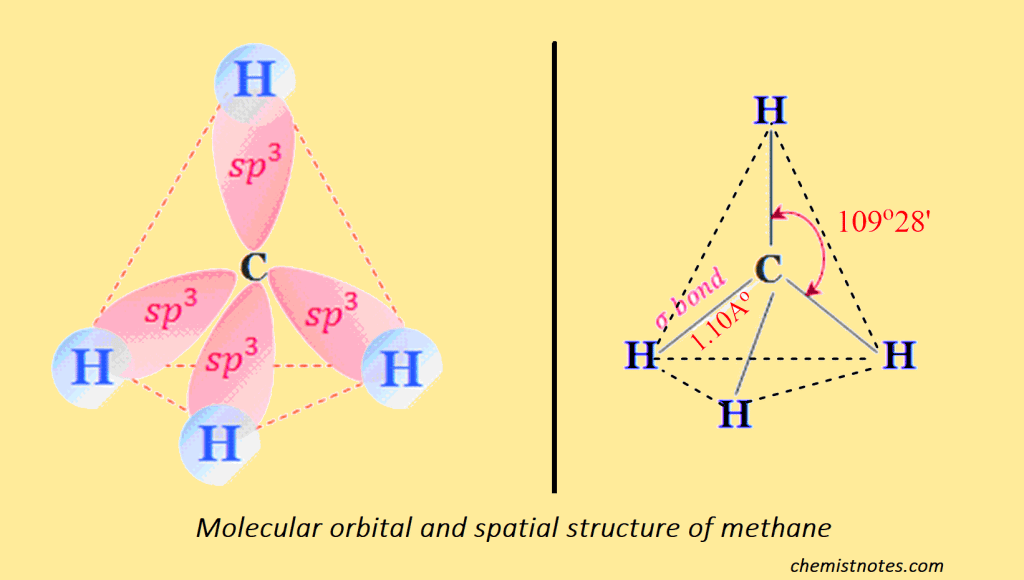
Saturated alkanes are made up of carbon and hydrogen. In alkane, each carbon atom is bonded with another carbon and hydrogen with a single covalent bond (sigma-bond) satisfying the four valencies of each carbon. In alkane, the carbon is sp3 hybridized and it forms the strong sigma covalent bond with the other four atoms or groups by using an sp3 hybridized orbital giving rise to the tetrahedral structure. The hybridization of carbon in alkane can be shown in the figure.
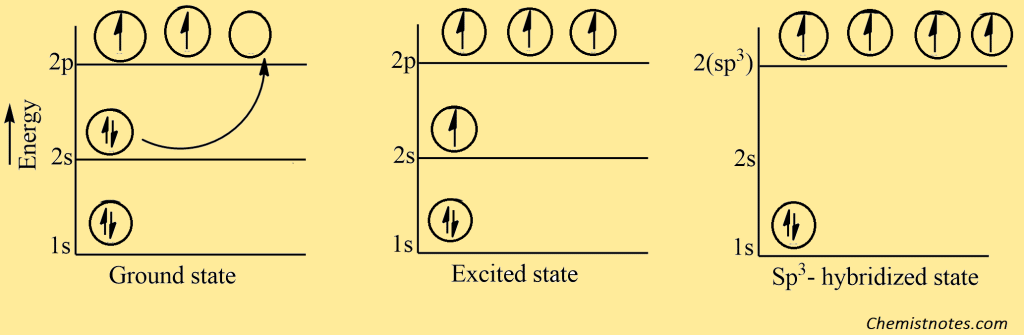
In alkane, a carbon forms four identical bonds with four other atoms and for this, it must contain a set of four equivalent orbitals. This can be achieved if a 2s and three 2p orbitals (Px, Py, and Pz) in the excited state are mixed or hybridized to give four new equivalent orbitals. These orbitals are known as sp3 hybrid orbitals. The mixing of a pure s-orbital with three p-orbitals is rather like mixing a gallon of pure red paint with three gallons of white paint to give four gallons of pink paint.
The four new sp3 orbitals obtained above are identical (same energy and shape) but differ only in their orientation in space with respect to each other. They are oriented as the four corners of the regular tetrahedrons. This alkane has a tetrahedral structure. In alkane, the carbon atom is bonded with other carbon atoms by a sigma covalent bond, and the bond length between carbon and carbon is 1.53 Ao, and carbon and hydrogen are 1.10Ao with a bond angle between any pair of bonds 1090 28′ as shown below:
Classification of carbon and hydrogen
It has been found extremely useful to classify each carbon and hydrogen atom in alkanes with respect to the number of other carbon atoms to which it is attached.
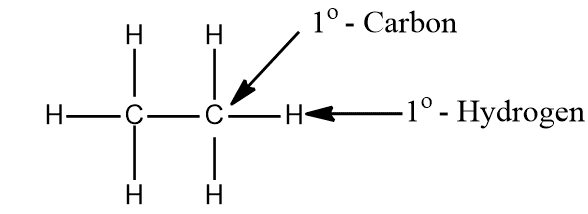
i. A primary carbon atom (10) is that carbon that is attached to only one other carbon and the hydrogen atom attached to it is called primary hydrogen (10 hydrogen).
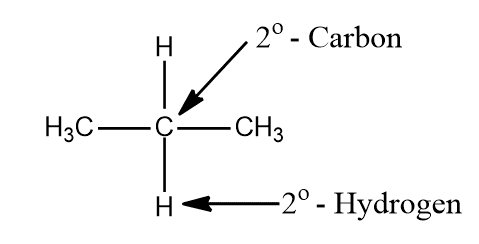
ii. A primary carbon atom (10) is that carbon that is attached to two other carbon atoms and the hydrogen atom attached to it is called secondary hydrogen (20 hydrogens).
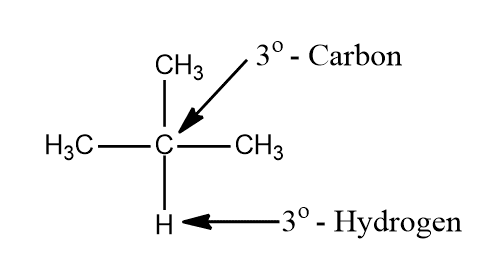
iii. Tertiary carbon (30 carbon) is that carbon which is attached to three other carbon atoms and hydrogen attached to it is called tertiary hydrogen (30-hydrogen)
iv. Quarternary carbon (40) is that carbon which is attached to four other carbon atoms.
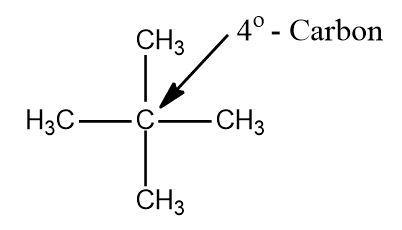
Nomenclature
Saturated hydrocarbon forms a homologous series.
Common system: In this system first four members of this series are known as methane (CH4), ethane(C2H6), propane(C3H8), and butane(C4H10). The rest of the members are named by prefixing greek numerals indicating the number of carbon atoms in the molecules to the suffix ‘ane’. Thus higher alkanes are named pentane (C5H12), hexane(C6H14), heptane(C7H16), etc.
| Molecular formula | Name | Molecular formula | Name |
| CH4 | methane | C10H22 | decane |
| C2H6 | ethane | C11H24 | undecane |
| C3H8 | propane | C12H26 | dodecane |
| C4H10 | butane | C13H28 | tridecane |
| C5H12 | pentane | C20H42 | eicosane |
| C6H14 | hexane | C30H62 | triconatane |
| C7H16 | heptane | C40H82 | tetracontane |
| C8H18 | octane | C60H122 | hexacontane |
| C9H20 | nonane | C94H190 | tetranonacontane |
IUPAC system
Step I: Name the longest carbon chain; The longest continuous carbon chain is selected as the basis for the name.
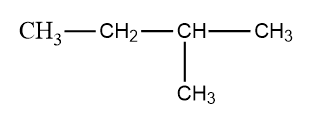
The largest continuous chain has four carbon. Thus the compound is named as butane.
Step II: Number the longest chain; The carbon atoms in the longest chain are numbered. The numbering is started from that end, which will number having the lowest value to carbon-carrying substituent.
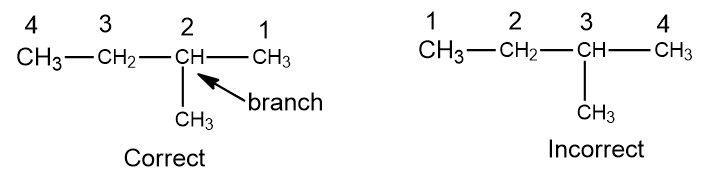
Step III: Locate and name the substituents; Each substituent is named, and the position of each substituent is indicated by the number of the carbon atom to which it is attached.

The attached group is located on carbon 2 of the chain and it is the methyl group.
Step IV: Combines the largest chain and substituents into the name. The position and the name of the substituent are added to the name of the longest chain and written as one word.
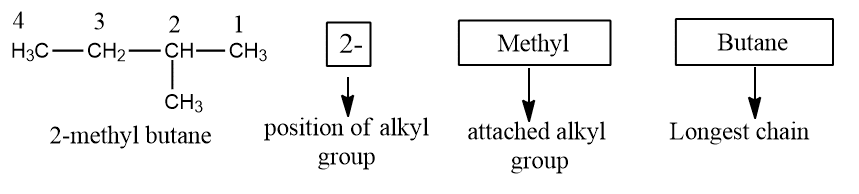
Additional steps are needed when more than one substituent is attached to the longest chain.
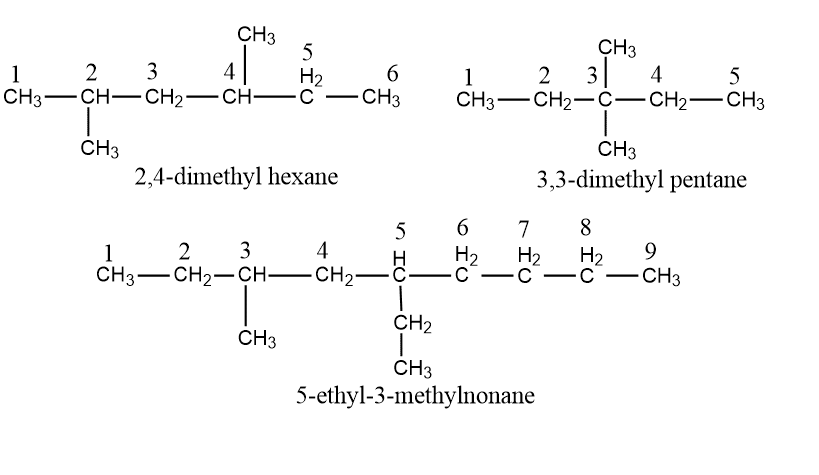
Step V: Indicate the number and position of substituents: If the same substituent is present in two or more times in the molecule, the number of this substituent is indicated by a prefix di-, tri-, tetra-, Penta- etc., and the location of each is indicated by a separated number. These position numbers separated by commas are put just before the name of the substituent with a hyphen before and after the numbers when necessary.
Physical properties of alkane
- The alkanes containing up to four carbon atoms (e.g. methane, ethane, propane, and butane) are colorless, odorless gases while pentane to heptadecane (C5-C17) is colorless, odorless liquids. Higher alkanes are waxy solids that are also colorless, and odorless.
- Alkanes are nonpolar molecules and almost insoluble in polar solvents like water and alcohol etc. but soluble in most nonpolar solvents like petrol, ether, benzene, etc.
- Their physical constants like boiling point, melting point, density, etc. increase with the increase in the number of carbon atoms, whereas solubility decreases with an increase in molecular weight.
- Alkanes are lighter than water. Their density increases with the rise of molecular weight.
- The boiling point of an alkane having a branched-chain structure is lower than its isomeric normal-chain alkane. This is because the spherical shape of branch chain alkane has a small surface area thus intermolecular forces are very low.
- Alkanes with an even number of carbon atoms have higher melting points than the alkane having a carbon atom less or the next higher alkane having an odd number of carbon atoms. Alkanes with an even number of carbon atoms possess a more symmetrical crystal pattern and are more firmly packed in the solid state than alkanes with an odd number of carbon atoms to permit greater intermolecular attraction, which in turn results in higher melting points.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.