Table of Contents
ToggleArenium Ion mechanism and its examples in organic chemistry are discussed here.
Arenium Ion Mechanism
The arenium ion mechanism is an aromatic electrophilic substitution mechanism that involves resonance stabilized carbocation, arenium ion intermediate. It takes place in two steps.
The first step involves the attack of an electrophile, which produces the arenium ion, a resonance stabilized carbocation intermediate, also known as the Wheland intermediate. Although resonance stabilizes the Wheland intermediate or σ-complex, now commonly known as the arenium ion, this step is accompanied by the loss of aromaticity, so the activation energy is high. In the second step, the leaving group departs. The first step is the rate-determining step and follows second-order kinetics.
A positive ion-dipole could be the attacking electrophile. The arenium ion is stabilized by resonance, but its resonance energy is substantially lower than that of the parent aromatic system due to the loss of aromaticity. As a result, the arenium ion loses a proton and reverts to an aromatic state, which is more stable. The elimination of the proton is aided by the presence of a base in the reaction mixture.
The reaction can be represented as:
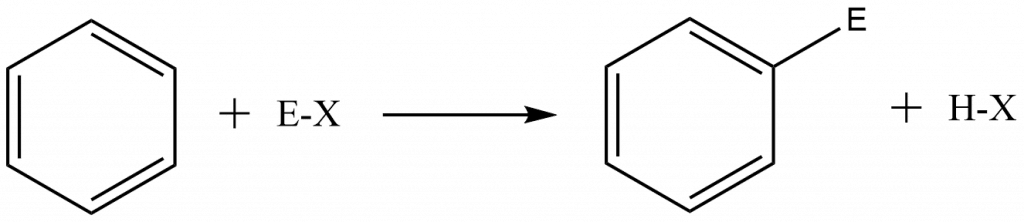
As mentioned above, the arenium ion mechanism takes place in two steps:
Step 1: Attack of the electrophile to the aromatic ring to give carbocation i.e arenium ion. This step is slow, therefore, is a rate-determining step.
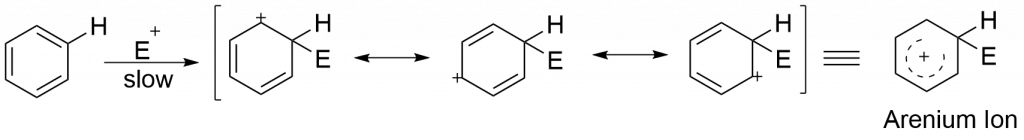
Step 2: Nucleophilic part of the reagent abstract proton from benzene ring to give the substituted product.
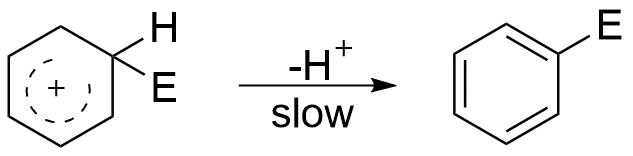
Energy profile diagram of arenium Ion
Arenium Ion mechanism video
FAQs/MCQs
What is arenium ion?
Arenium ion is the resonance-stabilized carbocation intermediate of electrophilic aromatic substitution of arenes.
Is arenium ion a less reactive intermediate?
Arenium is a cyclohexadienyl cation and appears as a very reactive intermediate in electrophilic aromatic substitution reactions.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






