Table of Contents
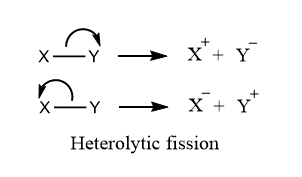
ToggleCarbocation and carbanions are the intermediates of reaction mechanisms formed due to unequal breaking of covalent bonds. In Heterolytic fission, shared pair of electrons are retained/taken by any one of the fragmented atoms and hence leads to the formation of positively charged species or carbocations, and negatively charged species or carbanions.
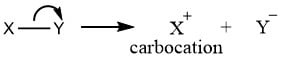
Carbocation
Carbocations are reaction intermediates that have carbon atoms containing six electrons and having a positive charge. It is also called carbonium. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms (that one which possesses greater electronegativity) retains a shared pair of electrons acquires a negative charge, while the remaining atoms that do not retain electrons acquire a positive charge. This positive-charged intermediate is carbocation intermediate or carbonium ion.
Some of the features of carbocations are:
- sp2 carbocation hybridization
- Geometry of carbon atom is triagonal planar
- Highly reactive i.e. less stable
- Carbocation shows paramagnetic behaviour due to incomplete electron pairing
- Acts as electrophile
- Acts as Lewis Acid
Carbocation examples: CH3+, CH3CH2+,(CH3)2CH+, (CH3)3C+, and so on.
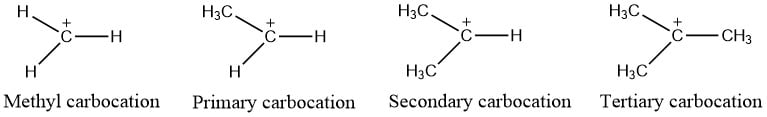
Types of carbocation
Depending on the number of carbon groups attached to the carbon atom containing positive charge, carbocations are of the following types:
- Methyl Carbocation: In this types of carbocation, no any carbon group is attached to the carbon atom containing positive charge, and is the least stable carbocation.
- Primary carbocation: One carbon is attached to the carbon atom containing positive charge.
- Secondary Carbocation: Carbon containing positive charge is attached to two other carbon atom
- Tertiary Carbocation: Positively charged carbon atom is bonded to 3 other carbon atoms. It is the most stable carbocation.
Carbocation stability:
Carbocation stability order: Methyl carbocation < primary carbocation < secondary carbocation < tertiary carbocation
Stability of carbocation
- Directly proportional to +I effect i.e. greater the number of alkyl groups, greater is the stability of carbocation. Example: CH3+< CH3CH2+<(CH3)2CH+< (CH3)3C+
- Directly proportional to hyperconjugation effect [stability = 1o < 2o < 3o carbocation
- Stability of carbocation increases by the resonance effect
- Carbocation with aromaticity possesses greater stability
Carbanion
Carbanions are reaction intermediates that have carbon atoms containing eight electrons and have a negative charge. It generally possesses 3 bonds and a lone pair of electrons. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms, that one which possesses greater electronegativity retains a shared pair of electrons acquires a negative charge. This negatively charged intermediate is the carbanion intermediate.
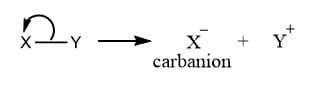
Some of the features of carbanion are:
- sp3 carbanion hybridization
- Geometry of carbon atom is pyramidal
- Highly reactive i.e. less stable
- Carbanion shows diamagnetic behaviour due to complete electron pairing
- Acts as nucleophle
- Acts as Lewis Base
Carbanion examples: CH3–, CH3CH2–,(CH3)2CH–,(CH3)3C–, and so on.
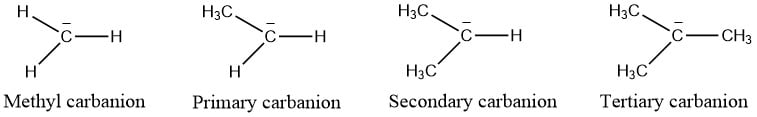
Types of Carbanion
Depending on the number of carbon groups attached to the carbon atom containing negative charge, carbanions are of the following types:
- Methyl Carbanion: In this types of carbocation, no any carbon group is attached to the carbon atom containing negative charge, and possess most in terms of stability.
- Primary carbanion: One carbon is attached to the carbon atom containing negative charge.
- Secondary Carbanion: Carbon containing negative charge is attached to two other carbon atom
- Tertiary Carbanion: Negatively charged carbon atom is bonded to 3 other carbon atoms. It is the least stable carbocation.
Carbanion stability:
Carbanion stability order: Methyl carbanion > primary carbanion> secondary carbanion > tertiary carbanion
Stability of carbocation
- Directly proportional to -I effect i.e. lesser the number of alkyl groups, greater is the stability of carbocation. When an alkyl group is linked to the carbanions (negatively charged carbon atom), it prefers to discharge electrons toward the carbon, and destabilizes the carbanions by increasing the intensity of the negative charge on the carbon. Example: CH3–>CH3CH2–>(CH3)2CH–>(CH3)3C–
- Stability of carbocation increases by the resonance effect and the aromaticity
- Carbocation with more s-character possesses greater stability
Differences between Carbanion and Carbocation
| Carbanion | Carbocation |
| Carbanions are reaction intermediates that have carbon atoms containing eight electrons and have a negative charge. | Carbocations are reaction intermediates that have carbon atoms containing six electrons and have a positive charge. |
| It is sp3 hybridized. | It is sp2 hybridized. |
| The geometry of the carbon atom is pyramidal | The geometry of carbon atom is trigonal planar |
| Shows diamagnetic behaviour due to complete electron pairing. | Shows paramagnetic behaviour due to incomplete electron pairing. |
| Acts as nucleophile | Acts as electrophile |
| Stability order: Methyl carbanion > primary carbanion> secondary carbanion > tertiary carbanion | Stability order: Methyl carbocation < primary carbocation < secondary carbocation < tertiary carbocation |
FAQs
Vinylic carbocation
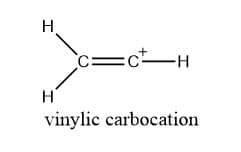
Vinylic carbocation is defined as a carbocation in which the positive-charged carbon is connected to a double bond.
Benzylic carbocation
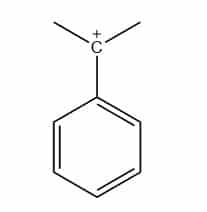
If the carbon atom having a positive charge is bonded next to a benzene ring, then it is benzylic carbocation.
Carbocation rearrangement
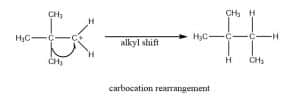
Carbocation rearrangement is described as the transfer of a carbocation from an unstable to a more stable state inside the molecule by the application of structural reorganizations, called “shifts.” The rearrangement/ structural reorganization is done either by hydride shift or alkyl shift.
Which is the most stable carbocation?
Depending on the number of carbon groups attached to the carbon atom containing positive charge, tertiary carbocation is the most stable.
least stable carbocation
Among the types of carbocation depending on the number of carbon groups attached to the carbon atom containing positive charge, primary carbocation is the least stable carbocation.
Carbocation formation
When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms (that one which possesses greater electronegativity) retains a shared pair of electrons acquires a negative charge, while the remaining atoms that do not retain electrons acquire a positive charge. This positive-charged intermediate is carbocation intermediate or carbonium ion.
Why tertiary carbocation is more stable?
Tertiary carbocation is the most stable in comparison to secondary, primary, and methyl carbocation. It is due to the Hyperconjugation effect in which localization of σ electrons of C-H bond of an alkyl group attached to an atom with an unshared p orbital takes place. Thus, C-H bond of carbon group attached to carbon atom containing a positive charge stabilizes by donating electron density into a carbocation’s empty p-orbital. Since it causes the dispersal of positive charge, the stability of carbocation is increased. Therefore, the greater the alkyl group gets attached to the carbocation, the more will be the hyperconjugation effect, and hence greater will be the stability.
Hybridization of carbocation and carbanion
Hybridization of the carbocation is sp2 while that of carbanion is sp3
Is carbanion paramagnetic?
No, carbanion shows diamagnetic behavior due to complete electron pairing
Formation of carbanion
Carbanions are the reaction intermediates that have carbon atoms containing eight electrons and having a negative charge. It generally possesses 3 bonds and a lone pair of electrons. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms, that one which possesses greater electronegativity retains a shared pair of electrons acquires a negative charge. This negatively charged intermediate is the carbanion intermediate.
Allylic carbanion
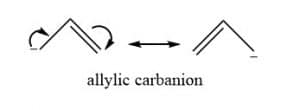
Allylic carbanion is formed when a carbon-carbon double bond is next to a carbon with a negative charge.
Vinylic carbanion
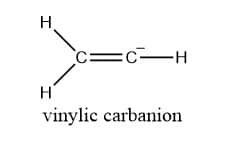
Vinylic carbocation is defined as a carbocation in which the negative-charged carbon is attached to a double bond.
Benzylic carbanion
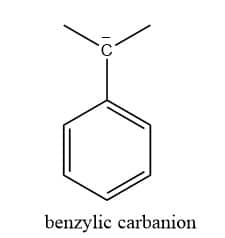
If the carbon atom having a negative charge is bonded next to a benzene ring, then it is benzylic carbanion.
Which is the most stable carbanion?
Depending on the number of carbon groups attached to the carbon atom containing negative charge, primary carbocation is the most stable.
Reactivity order of carbocation
Since reactivity is the reverse of stability, the reactivity order of carbocation is:
Reactivity order of carbanion
Since reactivity is the reverse of stability, the reactivity order of carbanion is:
Allylic carbocation
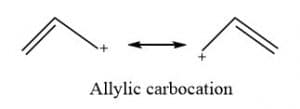
Allylic carbocation is formed when a carbon-carbon double bond is next to a carbon with a positive charge.






