Table of Contents
ToggleThe word ‘isomers’ actually means equal units. The development of coordination chemistry involves the presence of coordination compounds with the same formula but different ligand configurations. Isomers are two or more compounds that have the same formula but distinct atom configurations. Because isomers have different physical and chemical properties, it’s crucial to know which isomer we’re dealing with if there are multiple isomers. As we’ll see, coordination compounds have the same types of isomers as organic compounds, as well as a few that are distinct. Isomers are compounds that have the same molecular formula but various structural formulae, and they don’t always have the same characteristics. Stereoisomers, enantiomers, and geometrical isomers are among the various types of isomers.
Alfred Werner’s classification of isomerism of metal complexes.
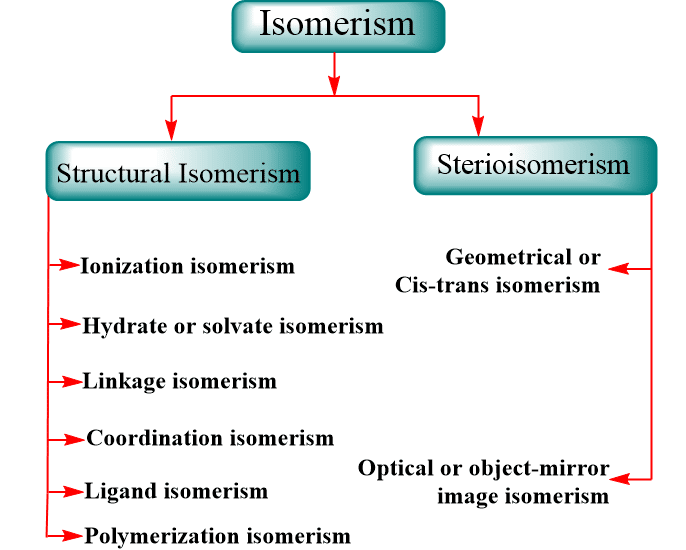
Structural Isomerism
Ionization isomerism
Ionization isomers are coordination compounds with the same molecular formula but different ions in solution. This property is known as ionization isomerism.
example:
[Co(NH3)5NO3]SO4 and [Co(NH3)5SO4]NO3
Hydrate or solvate isomerism
When a negative groups gets exchanged for a water molecule, the isomerism is called hydrate isomerism.
example:
[Co(Py)2Cl2(H2O)2Cl and [Co(Py)2Cl2(H2O)]H2O
Linkage isomerism
Two or more coordination compounds in which at least one of the ligands has a different donor atom are called linkage isomers (i.e., the connectivity between atoms is different). This type of isomerism can only exist with ambidentate or ambident ligand.
example:
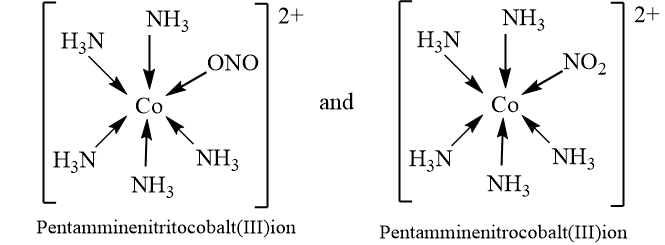
Coordination isomerism
When both positive and negative ions are complex ions, isomerism may be caused by the interchange of ligands between anion and cation.
example:
[Co(NH3)][Cr(CN)6] and [Cr(NH3)][Co(CN)6]
Ligand isomerism
Certain ligands can exist in isomeric forms. They can incorporated into complexes to give rise to isomers called ligand isomers.
example:
[Co(pn)2Cl2]Cl and [Co(tn)2Cl2]Cl
Polymerization isomerism
This is not true isomerism because it occurs between compounds having the same empirical formula but different molecular weights.
[Pt(NH3)2Cl2), [Pt(NH3)4][PtCl4], [Pt(NH3)4][Pt(NH3)Cl3]2 and [Pt(NH3)3Cl2[PtCl4]
Steriomerism
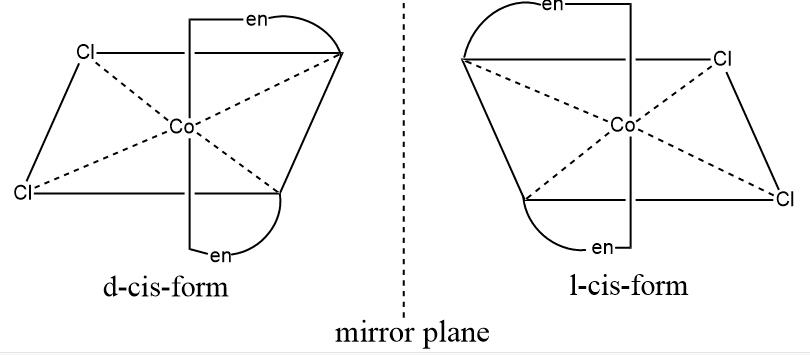
Geometrical or Cis-trans isomerism
In dsubstituted complexes, the substituted groups may be adjacent or opposite to each other, this give rise to geometric isomerism.
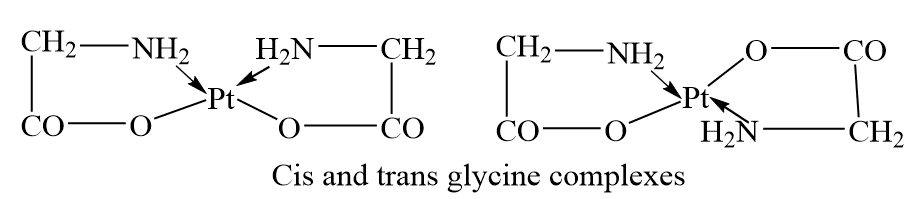
Optical or object-mirror image isomerism
Optical isomerism as associated with an enantiomeric pair.