Complex compounds that contain both a complex cation and a complex anion exhibit this form of isomerism. In this context, the ligand distribution between the two coordination spheres might change, giving rise to isomers known as coordination isomers. Coordination isomerism is obtained when some or all ligands of both the coordination spheres are interchanged with each other. This isomerism is illustrated by the following pairs of complexes. In these pairs, the central metallic atom in the two coordinate spheres (complex cation and complex anion) may be the same or different.
Examples of Coordination Isomerism
When both the positive and negative ions are complex ions, isomerism may be caused by the interchange of ligands between the anion and cation. For example, [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]. Intermediate types between these extremes are also possible.
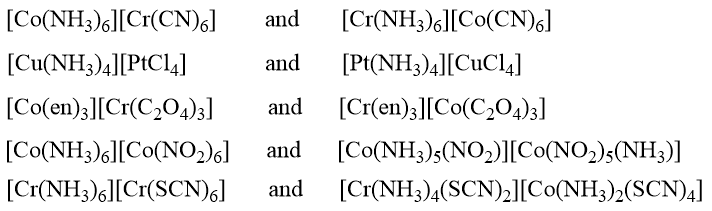
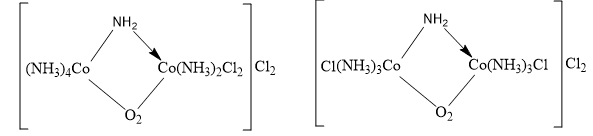
Coordination position isomerism
The difference in the position of ligands in a bridge complex causes a special type of coordination isomerism. This special type of isomerism is called co-ordination position isomerism. In polynuclear complexes, an interchange of ligands between the different metal nuclei gives rise to co-ordination positional isomerism.