Table of Contents
ToggleEffective atomic number concept was given by Sidgwick in 1923, which is also known as EAN. Sidgwick proposed that the center metal ions would receive electron pairs from donors until they had a sufficient number of electrons to give the metal in the complex ion the effective atomic number of the next inert gas element.
What is effective atomic number
According to Sidgwick’s concept, the ligands donate an electron pair to the central metal ion, resulting in the formation of a coordinate bond M-L, indicating that the ligand L has donated an electron pair to the metal ion, M. This idea is based on the fact that all ligands have at least one lone pair of electrons.
Sidgwick proposed that after the ligands have donated a certain number of electrons to the central metal ion via L-M bonding, the total number of electrons on the central atom, including those gained from the ligands in the bonding, is known as the effective atomic number (EAN) of the central metal ions.
The total number of electrons (EAN) surrounding the coordinated metal ions is often equal to the atomic number of the inert gas that follows the central metal atoms in the periodic table. This is known as the effective atomic number rule or the Noble gas rule. The rule is stated to be followed when the EAN is 36(Kr), 54(Xe), or 86(Rn).
Calculate effective atomic number
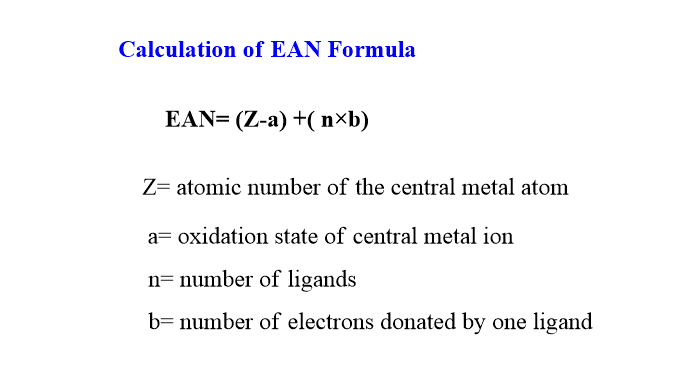
EAN of the central metal atom or ions in a given complex ion is given by the following expression.
EAN= (Z-a) +( n×b)
Where Z= atomic number of the central metal atom
a= oxidation state of central metal ion
n= number of ligands
b= number of electrons donated by one ligand
Therefore, in general, Effective nuclear number= (Atomic number of central metal atom- oxidation state of that metal) + total number of electrons donated by ligands
Example of calculation of EAN: [Fe(CN)6]-4
Here, z= 26, a=+2, n=6 and y= 2 Then,
EAN=(26-2)+ ( 6×2) = 36 ( Kr36)
Similarly, [Pd(NH3)6]4+, [Pt(NH3)6]4+, [Ag(NH3)4]+ follow EAN rule.
Many complexes don’t obey EAN rule i.e. in many complex ions, the EAN of the central metal is some units more or less than the atomic number of the next inert gas. For example: [Fe(CN)6]-3 , [Cr(NH3)6]4+,[Ni(NH3)6]2+ do not follow EAN rule.
Application of effective atomic number
The magnetic properties of complex ions can be predicted using the EAN rule. The complex ions whose center metal follows the EAN rule are diamagnetic. Here are several examples: The EAN of Co3+ion in [Co(NH3)6]3+ ion is equal to 36, indicating that this ion follows the EAN rule and so [Co(NH3)6]3+ ions are diamagnetic. This ion has been discovered to be diamagnetic. Sidgwick has also proposed that complex ions with central metal atoms that do not satisfy the EAN rule are often paramagnetic.
The difference between the EAN of the central metal atom and the atomic number of the inert gas that follows the central metal atom in the periodic table equals the number of unpaired electrons present in the complex ion. The magnetic moment (u) can be computed with the help of these unpaired electrons.
Furthermore, some central metal ions in [Ni(dmg)2]2+, [Pt(NH3)2Cl2]o, do not obey the EAN rule but are diamagnetic.
References:
- F.A. Cotton, G. Wilkinson, C.A. Murillo, and Manfred Bochmann Advanced Inorganic
Chemistry, (6th Edition), John Wiley and Sons, 1999






