Table of Contents
ToggleIn disubstituted complexes, the substituted groups may be adjacent or opposite to each other; this gives rise to geometric isomerism. Geometrical isomerism in octahedral complexes such as [Co(NH3)Cl2]+ exists in cis and trans forms. In an octahedral complex, the different coordination positions are numbered.
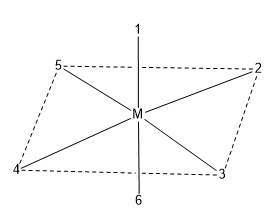
Along the 12 edges of the octahedran, there are 12 cis positions. They are (1,2), (1,3), (1,4), (1,5), (2,6), (3,6), (4,6), (5,6), (2,3), (3,4), (4,5) and (2,5). In order to avoid confusion, it is generally assumed that 1,2 positions are cis positions. There are three trans position (1,6), (2,4), and (3,5). It is generally understood that 1,6 positions are trans position.
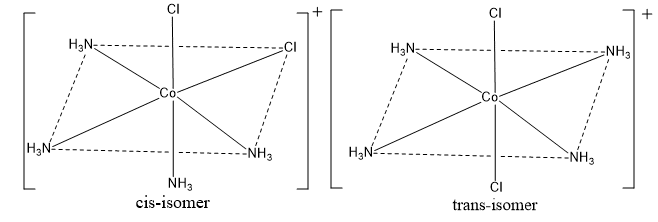
Octahedral complexes of the type [Ma4b2]
Where a and b are monodentate ligands, that exist as two geometrical isomers. For example: [Co(NH3)4Cl]+, [Pt(NH3)4Cl2]2+, [MCl2(NH3)4]
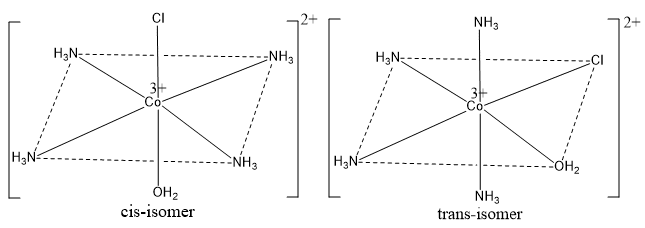
Octahedral complexes of the type [Ma4bc]
[Co3+(NH3)4(H2O)Cl]2+ ion is an important example of this type of complex. This ion has cis- and trans-isomers whose structures are shown in the figure below. In cis-form two NH3 molecules have cis positions to each other, and in trans-form these ligands (i.e. two NH3 molecules) have trans position with each other.
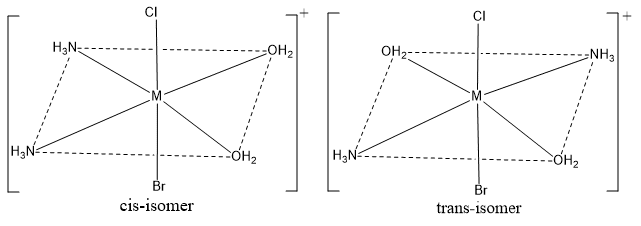
Octahedral complexes of the type [Ma2b2cd]
It exist as two isomers. Where, a,b,c and d are monodentate ligands. for example; [Co(NH3)2(H2O)2ClBr]+
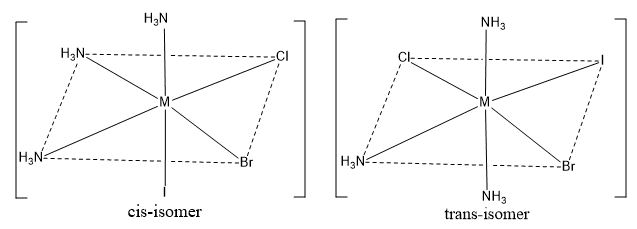
Octahedral complexes of the type [Ma3bcd]
For example: [Co(NH3)3ClBrI]
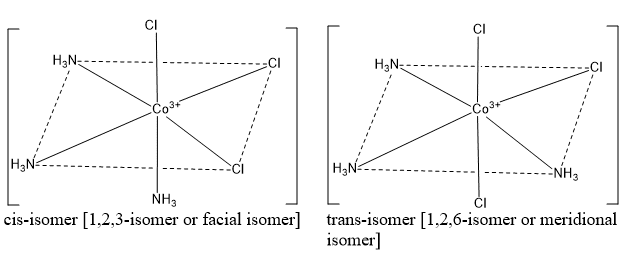
Octahedral complexes of the type [Ma3b3]
All complex of this type exist in cis- and trans- isomers. [Co(NH3)3Cl3], [Co(NH3)3(NO2)3], [Cr(H2O)3F3], [Cr(NH3)3Cl3] etc are important examples of octahedral complexes.
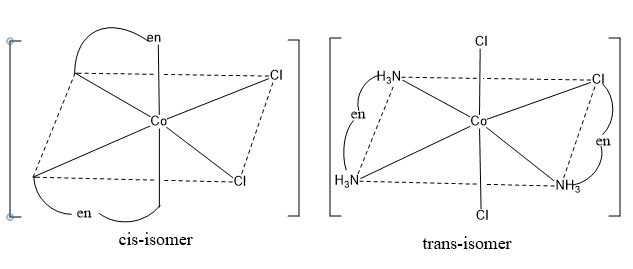
Octahedral complexes of the type [M(AA)2a2]
Here, AA is a symmetrical bidentate ligand such as ethylenediammine, and ‘a’ is a monodentate ligand exists as cis and trans isomers. Example: [Co(en2)Cl2]+.