If a molecule is asymmetric, it cannot be superimposed on its mirror image. The solutions of certain complexes rotate the plane of plane-polarized light either in the clockwise direction or in the anticlockwise direction. Such complexes are said to be optically active. They are known as optical isomers, and this phenomenon is called optical isomerism. Optical isomers have similar physical and chemical properties, but they differ in the direction in which they rotate the plane of plane-reflected polarised light. They are related to one another as objects and their mirror images. They are called either dextro or laevo ( d or l) depending on whether they rotate the plane of plane-polarized light in the clockwise or anticlockwise directions, respectively.
Conditions for Optical Isomerism
The molecule should be asymmetric, and the object and its mirror image should be non-superimposable over each other. Optical isomerism is common in octahedral complexes.
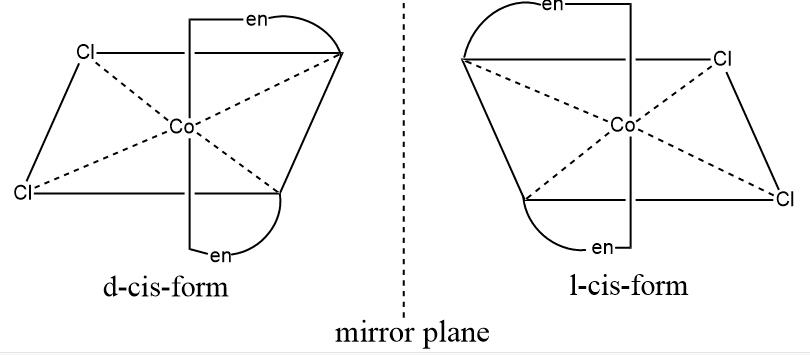
For example, cis [Co(en)2Cl2]+ exists as two optically active forms.
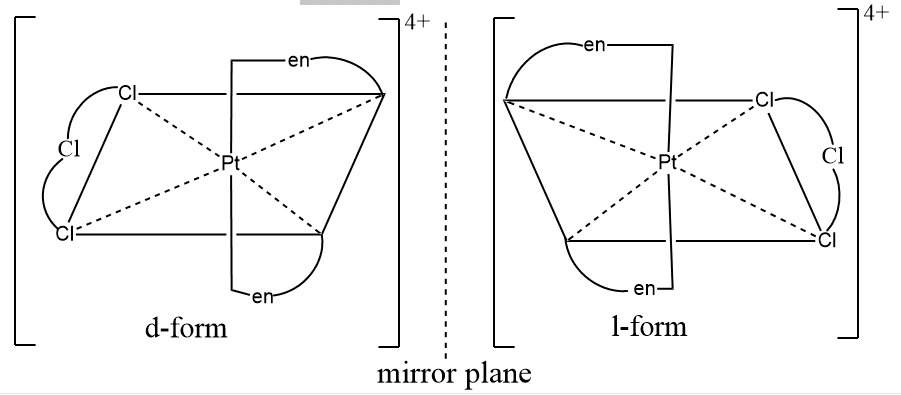
The presence of three bidentate ligands also causes asymmetry and hence leads to optical isomerism. For example, the tris(ethylenediamine) platinum (IV) ion, [Pt(en)3]4+ exists as the d and l forms.
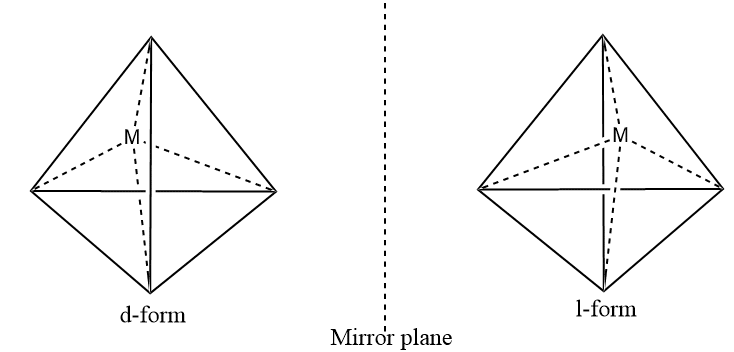
The tetrahedral complexes of the type [Mabcd] where a, b, c, d are monodentate ligands, exist as two optical isomers.
Tetrahedral complexes of Be2+, Zn+2, and other metals with unsymmetrical bidentate ligands, have been prepared and resolved into two optically active forms. For example, bis(benzoylacetonato)zinc (II), which is a tetrahedral complex, exists in two optically active forms.
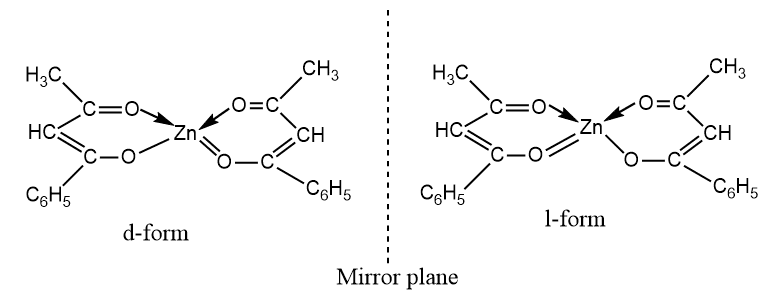
For the isomers to be resolvable, the chelate must be unsymmetrical with respect to the two ends of the chelating molecule. [Zn(acac)2] is not optically active because the two ends of the chelating molecules are symmetrical.






