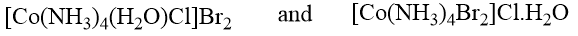Solvate isomerism is somewhat similar to ionization isomerism but involves the interchange of a solvent molecule with a negative ion. This type of isomerism is demonstrated by the three modifications of the composition CrCl3.6H2O. One modification does not lose water when stored over concentrated H2SO4 (as a dehydrating agent). Its molar conductance is of the same order as that of trivalent salt, indicating the presence of a tripoistive complex ion and three mononegative chloride ions. All the chloride content is immediately precipitated as AgCl by the AgNO3 solution. These properties of the isomers indicate their formula.
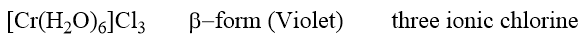
The second modification loses one mole of water per mole when stored over conc. H2SO4. Its molar conductance corresponds to that of a bivalent state, indicating the presence of a dispositive complex ion and two chloride ions. These properties correspond to the formula.
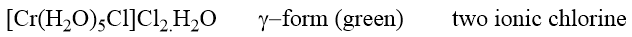
The third modification, based on its properties, is formulated as
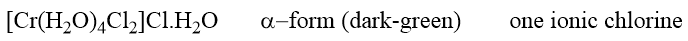
Examples of Solvate isomerism
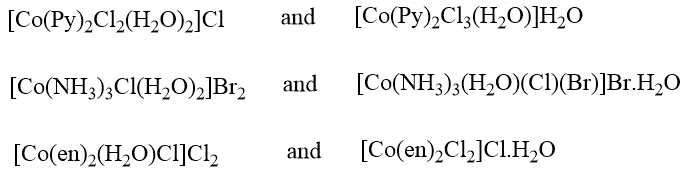
When a negative groups gets exchanged for a water molecule, the isomerism is more specifically called hydrate isomerism. An example involving both ionization and hydrated isomerism is provided by the following pair of complexes.