Table of Contents
ToggleBET adsorption isotherm proposed by Brunauer, Emmett, and Teller accounts for multilayer adsorption. According to the theory, adsorption takes place only on specific areas of the sample surface (one per molecule), and doesn’t stop at monolayer formation but first adsorbed gas molecules provide an adsorption site for subsequent gas molecules leading to the multilayer adsorption. i.e. after the monolayer has formed, the adsorption process proceeds to the formation of the multilayer forming second, third, and so forth layers. BET theory also considers that adsorption sites on the solid surface are homogenous and that adsorption at one site has no effect on adsorption at nearby sites.
Langmuir and Freundlich adsorption isotherm which fails to explain other isotherms except type I is well explained by the BET equation
BET Equation
On the basis of the above assumptions, BET proposed an equation called the BET equation.
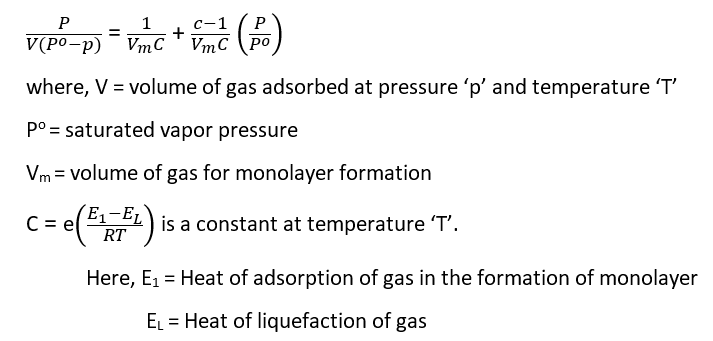
If E1>EL, type II adsorption isotherm is obtained but if E1<EL, then type III adsorption isotherm is obtained. When adsorption occurs in pores and capillaries, type IV and V adsorption isotherms are formed.
For type IV adsorption isotherm, E1must be greater than EL i.e. E1>EL
For type V adsorption isotherm, EL must be greater than E1 i.e. E1<EL
Applications of BET adsorption isotherm
Determination of the surface area of the adsorbent is one of the major applications of the BET equation.
BET Equation Video
FAQs/MCQs
What is adsorption isobar?
The graph obtained by plotting the extent of adsorption against temperature at constant pressure is known as adsorption isobar.
References
- Atkins, P. W.; De Paula, Julio; Keeler, James (2018). Atkins’ Physical chemistry (Eleventh ed.). Oxford, United Kingdom. ISBN 978-0-19-876986-6
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Brunauer, Stephen; Emmett, P. H.; Teller, Edward (1938). “Adsorption of Gases in Multimolecular Layers”. Journal of the American Chemical Society. 60 (2): 309 – 319. doi:10.1021/ja01269a023







One Response
Very very good