Table of Contents
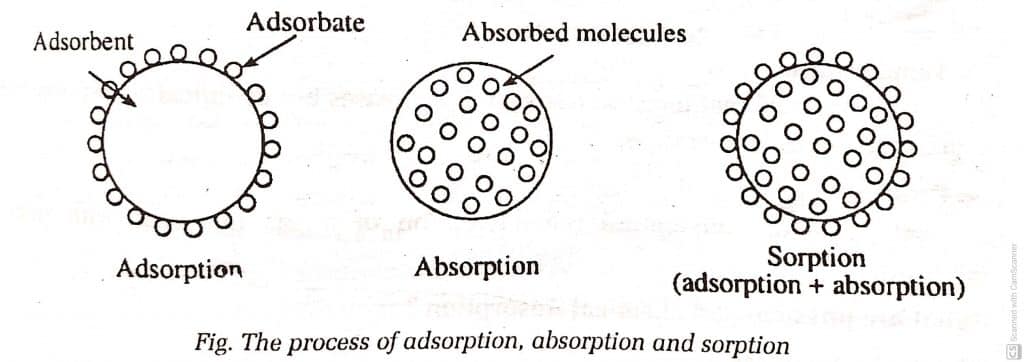
ToggleAdsorption is a phenomenon of an increase in the concentration of any substance or molecule on the surface of a solid or liquid. The substance which gets adsorbed or deposited is called adsorbate, while the substance on which adsorption/deposition takes place is called adsorbent. Some examples of adsorption include the adsorption of dye by charcoal, adsorption of gases by charcoal, adsorption of water on silica gel, and so on.
The phenomenon of an increase in the concentration of any substance or molecules on the bulk of another substance is called absorption. Sorption is a process that involves both adsorption and absorption phenomena simultaneously.
Adsorption Vs Absorption
| Adsorption | Absorption |
| The phenomenon of an increase in the concentration of any substance or molecule on the surface of a solid or liquid. | The phenomenon of an increase in the concentration of any substance or molecules on the bulk of another substance |
| It is a surface phenomenon. | It is a bulk phenomenon. |
| It involves less molecular interaction. | It involves greater intermolecular interaction |
| Exothermic process and spontaneous. | Endothermic process and non-spontaneous. |
| Adsorption is effective at low temperatures. | No effect of temperature on absorption. |
| Example: Adsorption of water vapor by silica gel. | Example: absorption of water by anhydrous CaCl2 |
Mechanism of Adsorption
The molecules or atoms that make up the majority of the adsorbent are symmetrically surrounded by other atoms or molecules. As a result, it will have no net attractive force. However, since the surface molecules are not symmetrically surrounded, they have some residual force due to the valence force. Adsorption occurs at the surface of the adsorbent due to the residual force remaining at the surface.
Why adsorption process is exothermic?
As we know, adsorption is a spontaneous process (ΔG = -ve). Thus from thermodynamics,
ΔG = ΔH – TΔS, where ΔH = change in enthalpy, ΔS = entropy change, T = absolute temperature
Entropy reduces when the adsorbate is adsorbed on the surface of the adsorbent, i.e. S = -ve. As a result, the previous equation becomes
ΔG = ΔH + TΔS
ΔH must be negative and greater than TΔS in order to get ΔG = -ve. Thus, the adsorption process is exothermic as ΔH is negative for the exothermic. process.
Types of Adsorption
- Physical Adsorption: Physical adsorption occurs when an adsorbate is kept on the adsorbent’s surface by a weak van der Waal’s force of attraction. Adsorption of oxygen on charcoal is an example of physical adsorption.
- Chemical Adsorption: Chemical adsorption occurs when an adsorbate is kept on the adsorbent’s surface by a strong chemical bond. Adsorption of hydrogen on nickel is one of the examples of it.
Physical adsorption and Chemical adsorption
| Physical adsorption (Physiosorption) | Chemical adsorption (Chemisorption) |
| Adsorbate is kept on the adsorbent’s surface by a weak van der Waal’s force of attraction. | Adsorbate is kept on the adsorbent’s surface by a strong chemical bond. |
| No new compounds are formed in this phenomenon. | Surface compounds are formed in chemisorption. |
| Decreases with a decrease in temperature. | Increases with an increase in temperature. |
| It is a reversible process. | It is an irreversible process. |
| It doesn’t require activation energy. | Activation energy is required. |
| Heat of adsorption is low (20-40 KJ mol-1) | Heat of adsorption is high (40-400 KJ mol-1) |
| Involves the formation of the multilayer. | Only a monolayer is formed. |
| The adsorption is effective at low temperatures and high pressure. | The adsorption is effective at high temperatures. |
| It is not specific in nature. | It is specific in nature. |
Factors affecting adsorption of gases by solids
Some of the factors affecting the rate of adsorptions are discussed below:
- Adsorption and Surface area of the adsorbent: Since adsorption is a surface phenomenon, the amount of adsorption is proportional to the area of the surface. The total amount of gas absorbed increases as the surface area of the adsorbent increases.
- Nature of Gas: The amount of gas that a solid can absorb is determined by the gas’s composition. In general, the more liquefiable a gas is, the easier it is to absorb it.
- Temperature: Physical adsorption decreases with an increase in temperature, while chemisorption increases with an increase in temperature.
- Pressure: The adsorption of gas increases with increasing pressure at a constant temperature.
Applications of Adsorption
- Cleansing of sugars
- Purification of water
- In adsorption indicator
- Used in preserving vacuum
- Separation of inert gases
- For chromatographic analysis
- Used in paint industries
- Applicable in Froth Flotation process
- For the removal of moisture and humidity
- Applicable in gas masks for removing poisonous gases by adsorption and thus purifying the air for breathing
Adsorption Video
References
- Atkins, P. W.; De Paula, Julio; Keeler, James (2018). Atkins’ Physical chemistry (Eleventh ed.). Oxford, United Kingdom. ISBN 978-0-19-876986-6
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Condon, James (2020). Surface Area and Porosity Determinations by Physisorption, Measurement, Classical Theory and Quantum Theory, 2nd edition. Amsterdam.NL: Elsevier.






