Hittorf’s rule is given by Scientist Hittorf in order to relate the relationship between the velocity of migrating ions and the concentration of ions around the electrode. The rule and the related mathematical expressions are described below. This rule is based on the concept of different ions moves at different speed hence the transport number varies with the velocity of migration of ions.
Hittorf’s rule
Electrolytes undergo ionization in solution to form positive and negative ions. When an electric current is passed through the electrodes of an electrolytic cell, the ions migrate to the opposite electrodes. i.e cation moves towards cathode and anion move towards the anode. Different ions have different rates of movement toward electrodes as different ions have different values of ionic mobility. Therefore, the speeds of cations moving towards the cathode and anions moving towards the anode are not always the same. The migration of ions can be illustrated by the following figure.
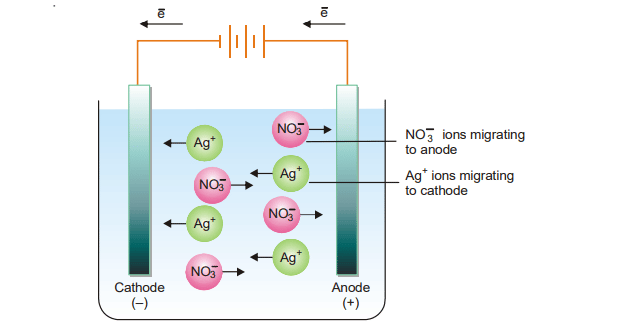
One important fact is that the speed of a cation traveling away from an anode is proportional to the decrease in the concentration of these ions at the anode. Similarly, the speed of an anion traveling away from the cathode will be proportional to the decline in anion concentration surrounding the cathode.
Hittorf studied a phenomenon of change in concentration of ions around electrode with a velocity of ions experimentally and gave a rule, known as Hittorf’s Rule. According to Hittorf’s rule, the decrease in the concentration of ions around an electrode is proportional to the velocity of migration of ions moving away from it.
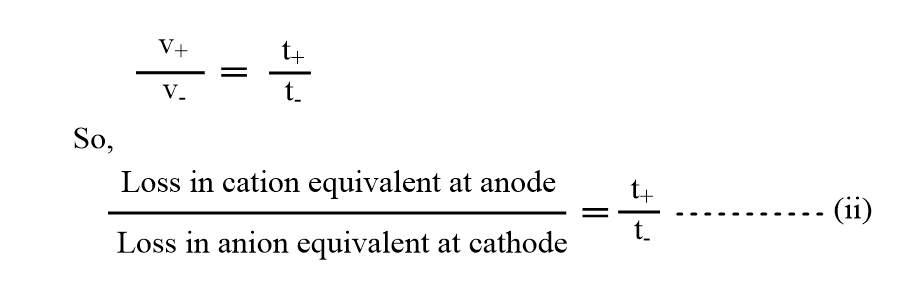
If V_ is the speed of anion and V+ is the speed of the cation, then Hittorf’s rule can be represented as:

But we know, Transport number(t) of ions is proportional to their absolute velocity(v). We can write
Now, adding 1 to both sides of equation(ii).
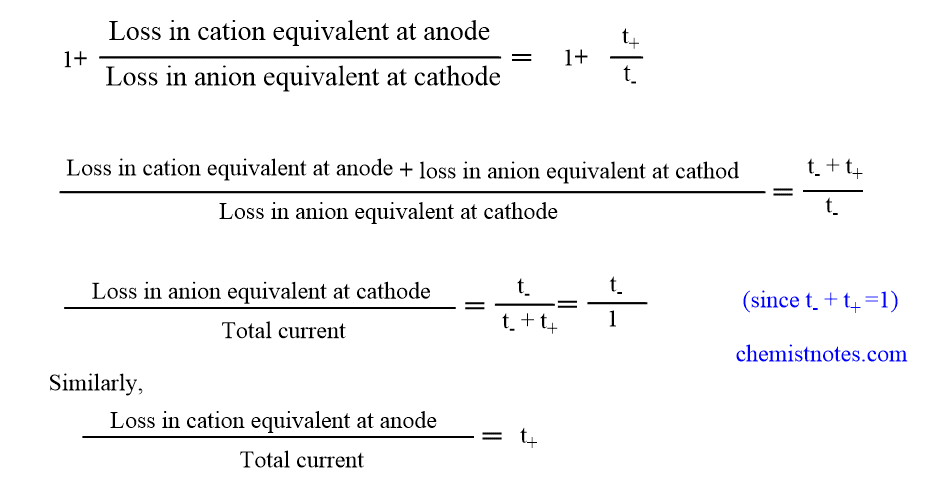
Thus, the fall in concentration around the electrode is directly proportional to the transference number of ion moving away from it. This is known as Hittorf’s rule
Hittorf’s rule video
References:
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012







One Response
Super method,easily understanding.