Table of Contents
ToggleElectrochemical series is the arrangement of electrode systems in order of increasing standard electrode potential or reduction potential. This series is based on experimental results which plays important role in choosing the right pair of electrodes for constructing an electrochemical cell. The definition and its application have been discussed below.
Define electrochemical series
The sequential vertical arrangement of various electrodes in the increasing order of standard reduction potential is known as the electrochemical series. This series is also known as electromotive series. The standard reduction potential(Eo) of metal is arranged in the order of increasing potential. The relative position of electrodes in the series can be used to predict the oxidizing or reducing ability of an electrode.
The electrochemical series table of metal electrodes is shown below:
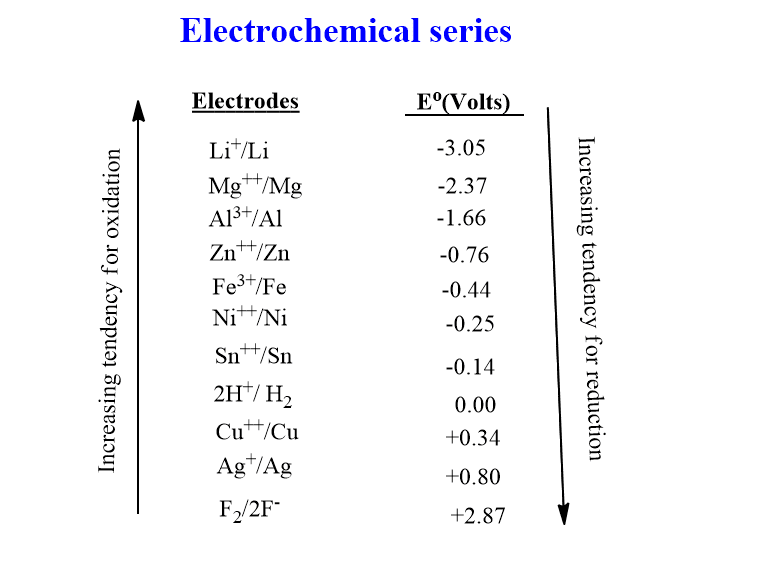
The magnitude of the standard electrode potential is a measure of the likelihood of reduction or oxidation. The reduced form of a half cell higher in the series has a larger tendency to donate electrons and be oxidized than the reduced form of a half cell lower in the series. In the same way, the oxidized form of a half cell lower in the series is more likely to accept electrons and be reduced than the oxidized form of a half cell higher in the series. Therefore, the best oxidizing agents are those present at the top of the table and the best-reducing agents are those present at the bottom of the series.
The above important points regarding the electrochemical series can be generalized as:
- The higher the value of the standard reduction potential(Eo), the better the oxidizing ability of the ion or molecule as it moves upwards along the series.
- The greater the negative value of Eo, the greater the capacity of the ions, elements, or compounds to reduce.
- Under normal conditions, any substance in the series will spontaneously oxidize any other substance in the series lower than it. It means the metals with higher electrode potential will be reduced by the system with lower electrode potential.
- The electrode having the greater value of standard reduction potential is taken as Cathode while the electrode with the lower value of standard reduction potential is taken as Anode, during the construction of the electrochemical cell.
Electrochemical series trick
The electrochemical series is difficult to remember but there is one easy trick to remember. Let’s see:
Lifeless Mysterious Aliens Zested Forks Nevertheless Tasty Hydras Chased Sweaters Frantically.
Application of electrochemical series
The electrochemical series have many applications and some of these have been discussed.
- To calculate the emf of a cell in the standard state.
- The net cell reaction is obtained by the sum of the anode and cathode reaction and the total emf of a cell is given by Eo(cell)= Eo(right)-Eo(left). If the emf of a cell is found to be positive, the net reaction is spontaneous in the direction shown. If the cell emf is found to be negative, the net cell reaction is spontaneous in the reverse direction.
- To predict the relative strength of the oxidizing and reducing agent
- To predict the metal displacement reaction
- The metals with more negative reduction potential can displace those with lesser negative or positive reduction potential from their solution. For example, the Eo of Zn++/Zn and Cu++/Cu are -0.76 v and +0.34 v respectively. The Zn can therefore displace Cu from the solution of CuSO4.
- To predict whether the metal reacts with the acid to liberate H2 gas.
- All the metals having negative standard reduction potentials show a greater tendency towards losing electrons as compared to hydrogen. So, when such a metal is placed in an acid solution the metal gets oxidized and the H+ ion is reduced to liberate H2 gas. Thus, all the metals having negative standard reduction potential can liberate H2 gas from an acid solution.
- To predict the spontaneity of redox reaction.
- If emf of a cell Eocell is found to be positive the net cell reaction is spontaneous (feasible) in the direction shown. If the emf of the cell is negative then the net cell reaction is non-spontaneous.
Electrochemical series video
Note: Don’t get confused with the Spectrochemical series and this electromotive series. These are two different series with different significances.






