Table of Contents
ToggleAn Adsorption isotherm is a relationship between a gas’s equilibrium pressure and the amount of gas adsorbed on a solid adsorbent at a fixed temperature. The extent of adsorption is determined by the temperature and saturated pressure or equilibrium pressure. A curve or plot generated by plotting the extent of adsorption against pressure at constant temperature is called an adsorption isotherm.
Adsorption Isotherm
The adsorbate gets adsorbed on the adsorbent in the process of adsorption.
The equilibrium direction would move in the direction where the stress can be reduced (Le-Chatelier’s principle). When an excess of pressure is applied to the equilibrium pressure, the equilibrium shifts in the direction of a decrease in the number of molecules. With an increase in pressure, the forward direction of equilibrium will be preferred because the number of molecules reduces in the forward direction.
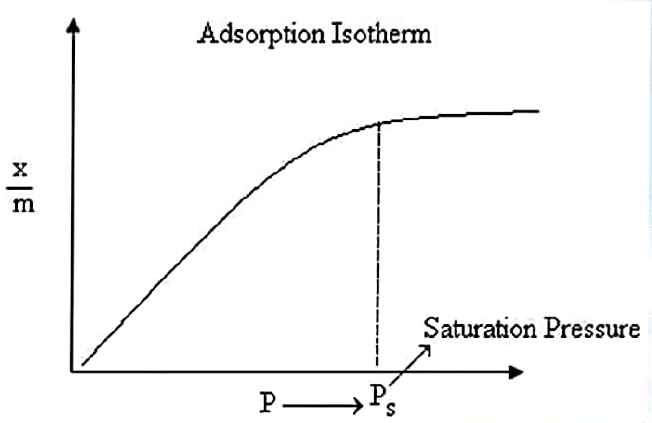
Adsorption stops after the saturation pressure Ps, as seen in the diagram. This can be explained by the fact that the adsorbent’s surface has a finite amount of vacancies. When the pressure is high enough, all of the sites are occupied, and a further increase in pressure has no effect on the adsorption process. Thus, adsorption is pressure independent at high pressures.
The adsorption phenomenon involves five different forms of adsorption isotherms, as shown in the diagram below. The Type I adsorption isotherm models occur in both physical and chemical adsorption and account for the formation of monolayers, whereas the other isotherms occur exclusively in physical adsorption and account for the formation of multilayers.
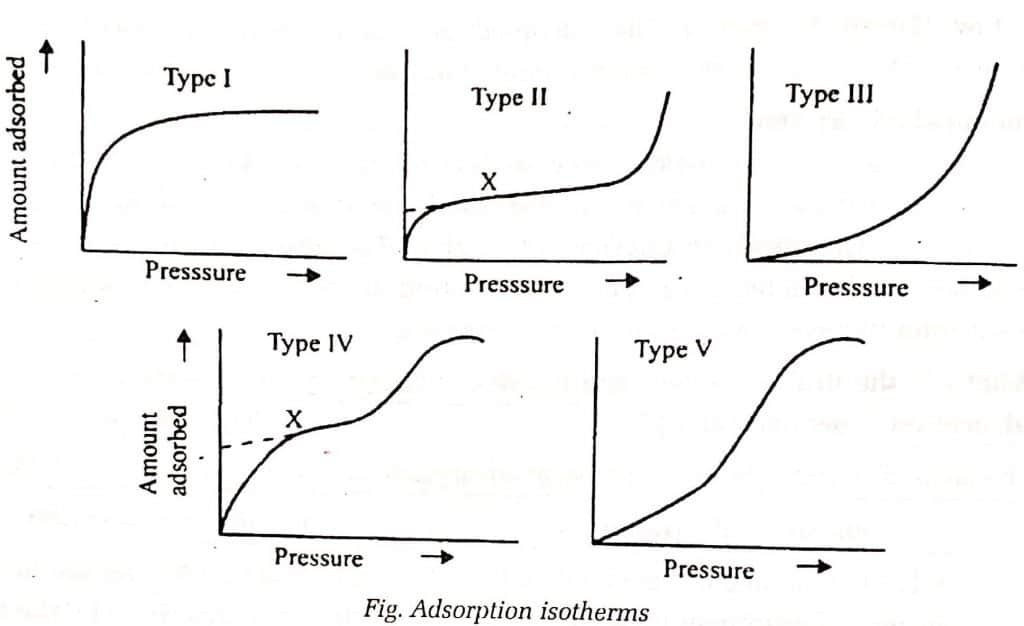
Type I adsorption isotherm
Type I isotherms are those in which the pore size is not substantially larger than the molecular diameter of the sorbate molecules. This isotherm shows that the extent of adsorption increases with pressure until it reaches saturation, at which point no further adsorption occurs.
Type II adsorption Isotherm
Isotherms of type II are generally observed in adsorbents that have a wide range of pore sizes i.e. macroporous. It is obtained when the bilayer is formed only after the monolayer has been fully formed, and the trilayer is formed only after the bilayer has been fully formed.
Type III adsorption Isotherm
It is obtained when the formation of monolayers, bilayers, trilayers, and other layers all take place at the same time, resulting in an almost exponential increase in the amount of adsorption.
Type IV adsorption isotherm
Type IV isotherm predicts the formation of two surface layers on the plane surface or on the wall of a pore much wider than the sorbate’s molecular diameter i.e.e mesoporous.
Type V adsorption isotherm
This type of adsorption isotherm is obtained only when intermolecular attraction effects are large, and adsorption takes place in pores and capillaries.
Various scientists have proposed several adsorption isotherms, including
Adsorption Isotherm Video
References
- Atkins, P. W.; De Paula, Julio; Keeler, James (2018). Atkins’ Physical chemistry (Eleventh ed.). Oxford, United Kingdom. ISBN 978-0-19-876986-6
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






