Table of Contents
ToggleFreundlich adsorption isotherm proposed by Herbert Freundlich in 1909 explains type I adsorption isotherm. Freundlich proposed an empirical relation between the extent of adsorption (quantity of mass adsorbed by unit mass of solid adsorbent) and pressure. This relation is known as the Freundlich equation or Freundlich adsorption isotherm.
Freundlich equation
The Freundlich equation can be represented as

From the above equation, Freundlich proposed the following type of curve
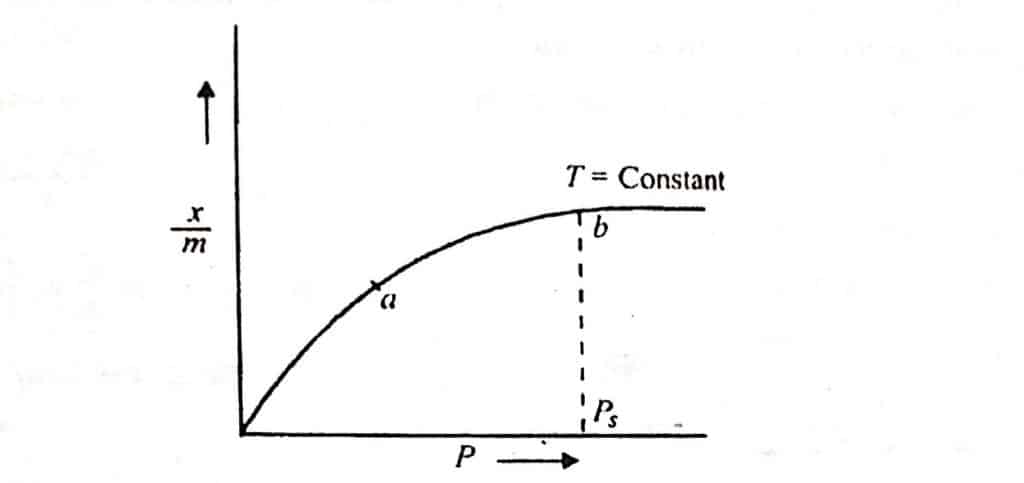
This plot indicates that at the low-pressure, extent of adsorption is directly proportional to pressure (adsorption increases with an increase in pressure) but at high pressure, the extent of adsorption is independent of the pressure.
Limitations of Freundlich adsorption isotherm
Freundlich’s adsorption isotherm fails to determine the extent of adsorption at higher pressures.
Freundlich adsorption isotherm video
References
- Atkins, P. W.; De Paula, Julio; Keeler, James (2018). Atkins’ Physical chemistry (Eleventh ed.). Oxford, United Kingdom. ISBN 978-0-19-876986-6
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Freundlich, Herbert (1907). ” Über die Adsorption in Lösungen.” Zeitschrift für Physikalische Chemie – Stöchiometrie und Verwandschaftslehre. 57 (4), 385–470.






