Table of Contents
ToggleA dipole moment can arise in any system in which there is a separation between positive and negative charges. Dipole moments can arise in ionic bonds as well as in covalent bonds. If the center of the negative charge does not coincide with the center of the positive charge, then the molecule is polar. Such a molecule constitutes a dipole: two equal and opposite charges separate in space.
Units and symbols of Dipole moment
A dipole is often symbolized by ⇸ where the arrow points from positive to negative. The dipole moment is denoted by μ and the dipole moment is measured in Debye units denoted by ‘D’. 1D=3.33564×106−30 C.m.
The formula for the dipole moment
The dipole moment occurs due to the difference in electronegativity between two chemically bonded atoms. The molecule possesses a dipole moment, μ, which is equal to the magnitude of the charge, e, multiplied by the distance, d, between the centers of charge:
μ = e ✕ d
μ in debye units, D
e in e.s.u.
d in cm.
In this way, it is possible to measure dipole moment, some of the values obtained by using this formula are listed in the table below. We shall be interested in the values of dipole moments as indications of the relative polarities of different molecules.
| Molecule | μ | Compound | μ | compound | μ |
| H2 | 0 | HF | 1.75 | CH4 | 0 |
| O2 | 0 | H2O | 1.84 | CH3Cl | 1.86 |
| N2 | 0 | NH3 | 1.46 | CCl4 | 0 |
| Cl2 | 0 | NF3 | 0.24 | CO2 | 0 |
| Br2 | 0 | BF3 | 0 |
Dipole moment and Polarity
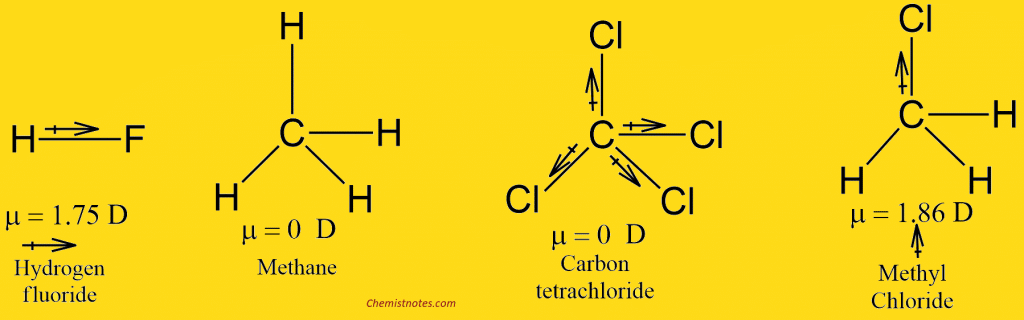
Molecules with zero dipole moments, such as H2, O2, N2, Cl2, and Br2 are non-polar. The two identical atoms in each of these molecules have the same electronegativity and share electrons equally; e is large, and hence μ is large too.
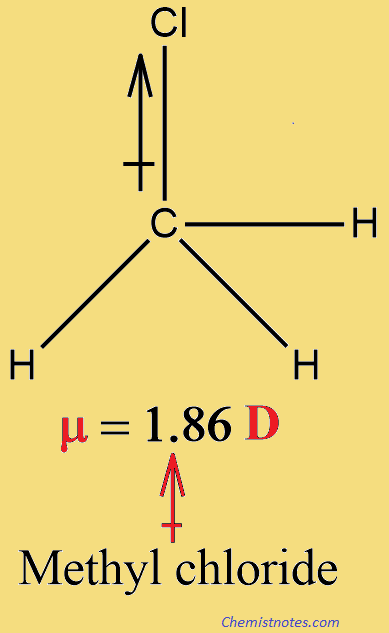
Methane(CH4) and carbon tetrachloride(CCl4), have zero dipole moments. We certainly would expect the individual bonds of carbon tetrachloride at least to be polar; because of the very symmetrical tetrahedral arrangement, however, they exactly cancel each other out. In methyl chloride, CH3Cl, the polarity of the carbon-chlorine bond is not canceled, however, and methyl chloride has a dipole moment of 1.86 D.
Thus, the polarity of a molecule is determined not only by the polarity of its individual bonds but also by the orientation of the bonds, i.e., the shape of the molecule.
Examples of Dipole Moments
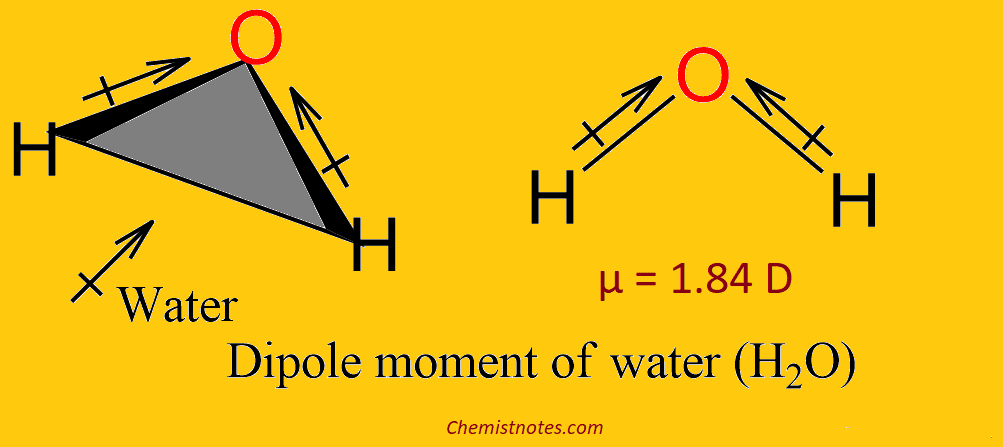
The dipole moment of the Water molecule (H2O)
The dipole moment of H2O is 1.84 D. In water, the dipole moment arises because oxygen is more electronegative than hydrogen; oxygen pulls in the shared electrons and increases the electron density around itself. This creates an electric dipole moment vector with a partial negative charge on the oxygen atom.
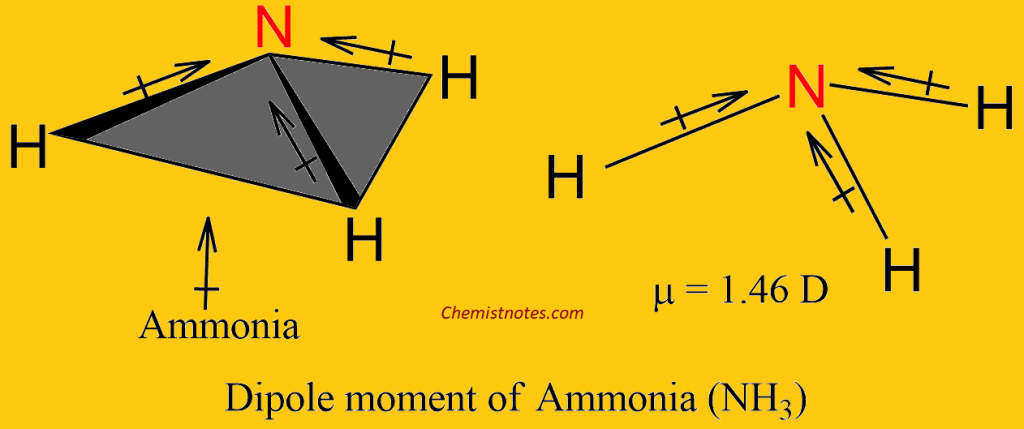
The dipole moment of the Ammonia molecule (NH3)
Ammonia has a dipole moment of 1.46 D. This could be accounted for as a net dipole moment resulting from the three individual bond moments and would be in the direction shown in the diagram below.
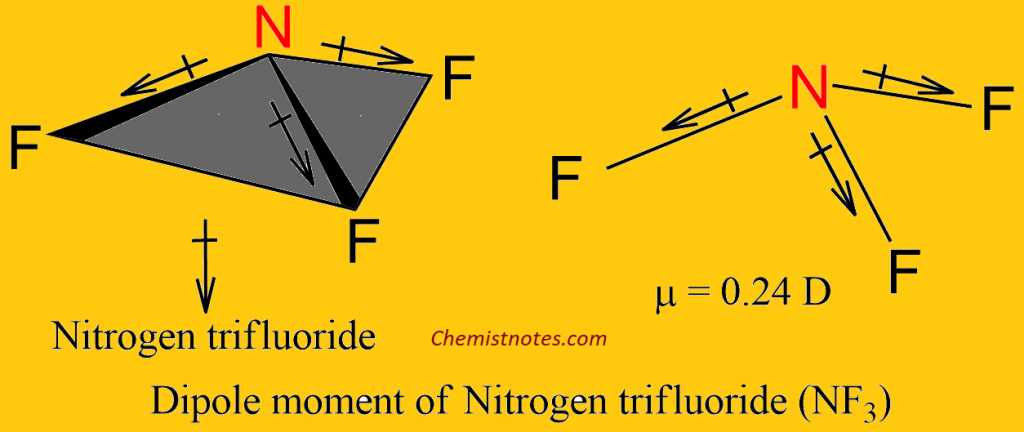
The dipole moment of the Nitrogen trifluoride (NF3)
The dipole moment of nitrogen trifluoride is 0.24 D. Fluorine is the most electronegative element of all and should certainly pull electrons strongly from nitrogen; the N-F bonds should be highly polar, and their vector sum should be large, far larger than for ammonia.
FAQs
What is a dipole moment?
A dipole moment can arise in any system in which there is a separation between positive and negative charges.
Which molecule has zero dipole moment?
H2, O2, N2, Cl2, and Br2 molecule has zero dipole moment.
What is the unit of dipole moment?
The dipole moment is measured in Debye units denoted by ‘D’.






