Table of Contents
ToggleChemical properties of alkenes mainly due to the presence of double bond. Alkenes are more reactive than alkanes because the π electrons of a double bond are located much farther from the carbon nuclei and are thus less firmly bound to them. The overlap of the atomic orbitals in forming a π-bond is not as effective as that in σ-bonds. Thus π-bond is weaker than sigma-bond and more easily broken and hence more reactive.
The addition reaction is the main characteristic reaction of alkene. A molecule with carbon-carbon double bonds can be considered both an electron-rich substrate and an electron-seeking species. Thus, the most important alkene reactions are electrophilic addition reactions.
Addition reactions of Alkenes
Alkenes undergo addition reactions with various reagents to give saturated compounds. In addition reaction, the π-bond is converted into a new σ-bond in the product.
Addition of Hydrogen (Catalytic hydrogenation)
The alkenes when heated with hydrogen gas in presence of metal catalyst Raney ni, pt, or pd to give saturated hydrocarbon is known as catalytic hydrogenation. For catalytic hydrogenation, high pressure and temperature are required.
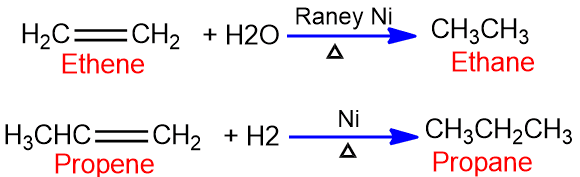
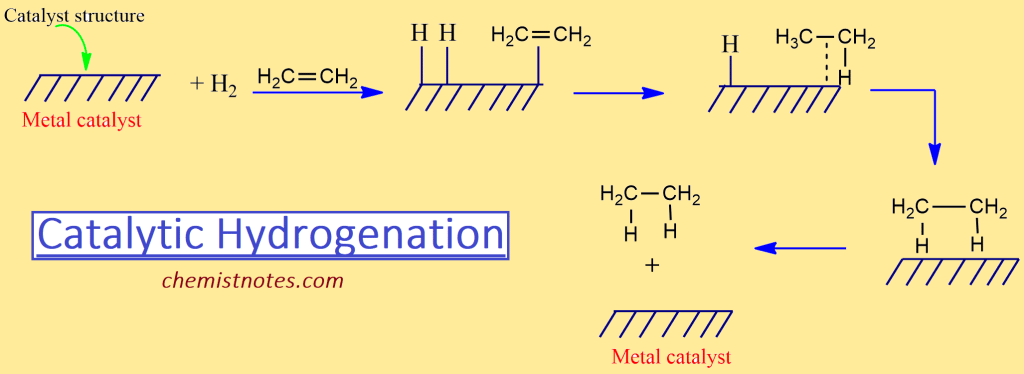
The reaction proceeds by an adsorption mechanism. The hydrogen is adsorbed on the metal surface along with alkene molecules when a hydrogen molecule and an alkene molecule lie next to each other their closeness and weakening of their bonding permit a rearrangement of electrons to form new bonds. The overall result of the catalytic hydrogenation is the addition of hydrogen to the carbon-carbon double bond to form alkane.
The addition of hydrogen molecule to the alkene can be summarized as follows:
Electrophilic Addition Reaction
Those organic reactions which are initiated by an electrophile are known as electrophilic addition reactions. They are characteristic reactions of alkenes. Halogens (Cl2, Br2) react with an alkene in the presence of an inert solvent to form diahalogens derivatives.
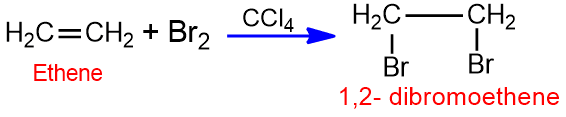
The addition of halogen to alkene does not take place by a carbonation mechanism but rather by bromonium ion mechanism. In this reaction, the Br2 molecule acts as a nucleophile.
Step I
In step I, the electrophile attacks the double bond to give a bromonium ion.
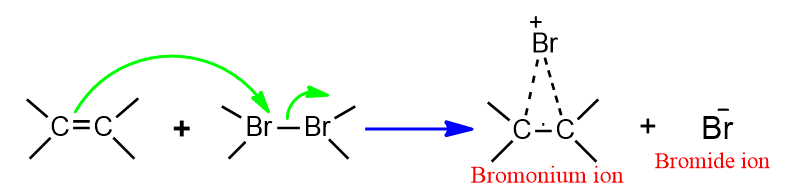
Step II
In second step the bromide ion attacks to this bromonium ion to give dibromoderivative. The overall result of the reaction is trans addition of bromine molecule to alkene.
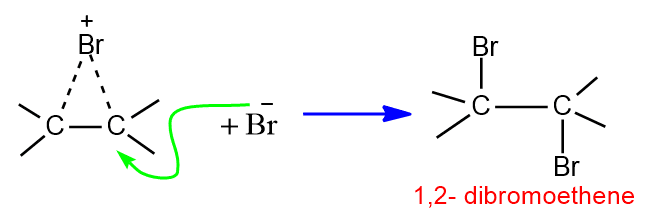
Addition of Halogen acids
When alkene adds to the halogen acid it gives alkyl halide i.e, addition of HBr to ethene.
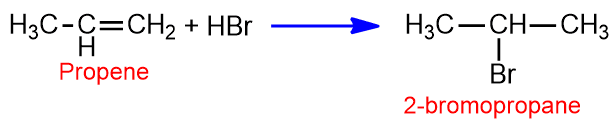
When an alkene is symmetrical about the double bond it gives only one product. When alkene is unsymmetrical, then the addition takes place according to Markovnikov’s rule.
The Russian chemist Vladimir Markovnikov (1869) studied a large number of addition reaction of unsymmetrical olefins observed that, when two isomeric products are possible one of them usually predominates. Then he put forwarded empirical rule known as Markovnikov’s rule. The rule states that ‘When an unsymmetrical reagent like HBr adds to the unsymmetrical alkene such as propene, the positive part of the reagent goes to that double bonded carbon which has greater number of hydrogen.’
For example: The addition of HBr to the propene gives 2-bromopropane but theoretically there is also possibility of formations of n- propyl bromide.
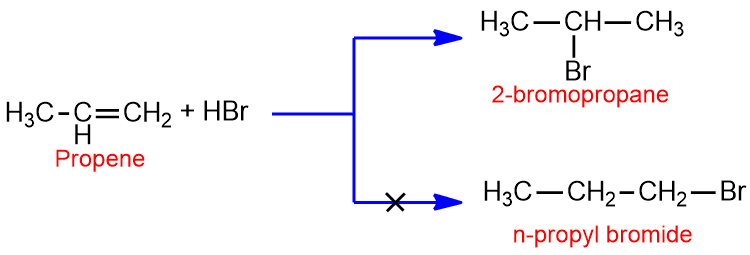
Markovnikov’s rule can be explained in terms of modern mechanistic theory. Consider the addition of HBr to propene.
Step I: HBr ionizes to give electrophile (H+) and nucleophile (Br–).

Step II: The electrophile H+ attacks the carbon-carbon double bond to give carbocation.
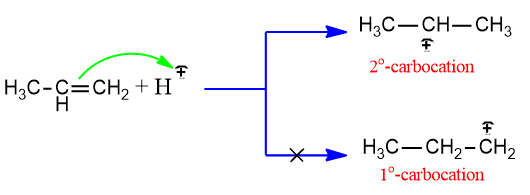
The stability order of carbonation is 3o > 2o> 1o. Thus we have, more stable the carbocation faster it is formed. Thus, in the above reaction, 2o carbonation is formed, which gives the product 2-bromobutane.
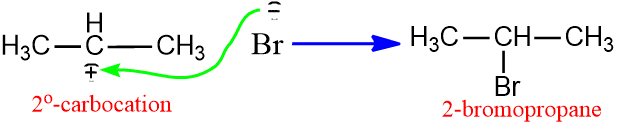
Addition of Water (Catalytic hydration)
Alkenes can be hydrated to alcohols in the presence of a diluted mineral acid catalyst. e.g., H2SO4, H3PO4.
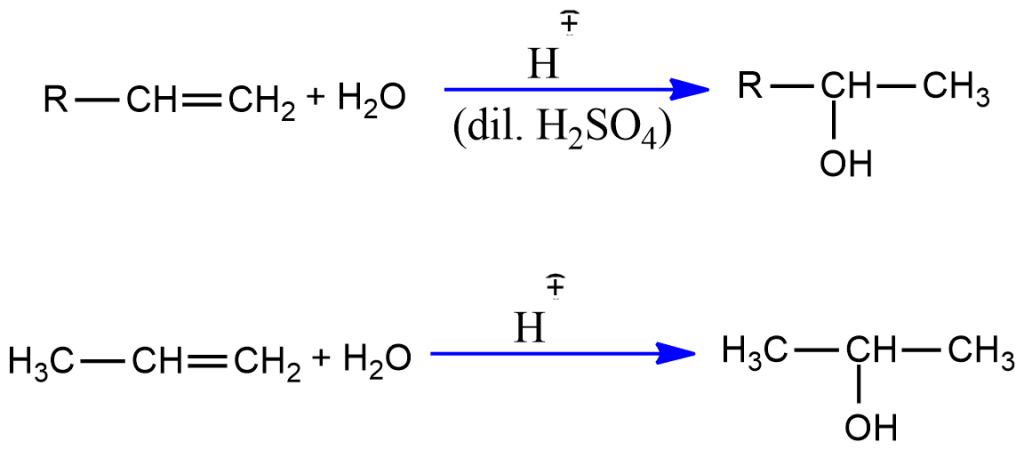
Unsymmetrical alkene gives the Markovnikov’s products.
Addition of sulphuric acid
Alkenes react with cold concentrated sulphuric acid to give alkyl hydrogen sulphate. Alkyl hydrogen sulphate further heated with H2O to give alcohol.
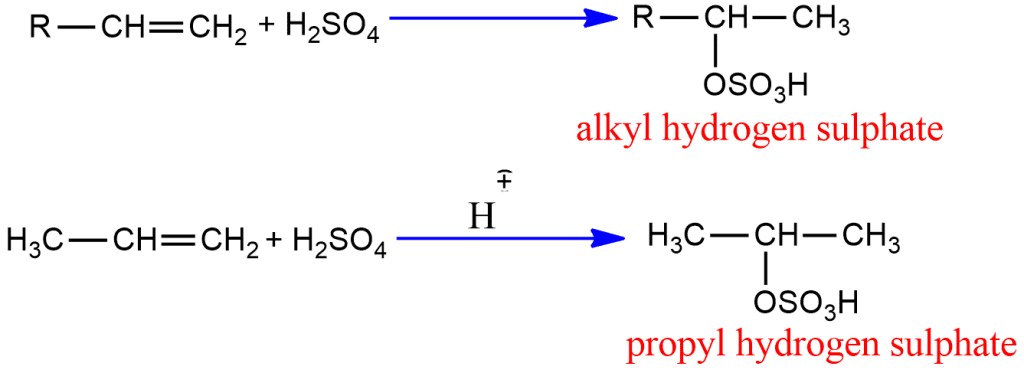
Unsymmetrical alkene follows Markovnikov’s addition.
Addition of Ozone (Ozonolysis)
When alkene reacts with ozone in inert solvent (ether, CCl4) it gives ozonide. On warming ozonide with Zn in water breaks down to give two molecules of carbonyl compounds (aldehyde or ketone). The process of formation of ozonoid and its decomposition to give carbonyl compound is called ozonolysis.
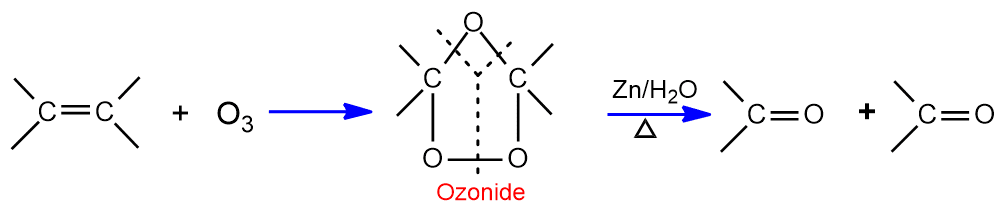
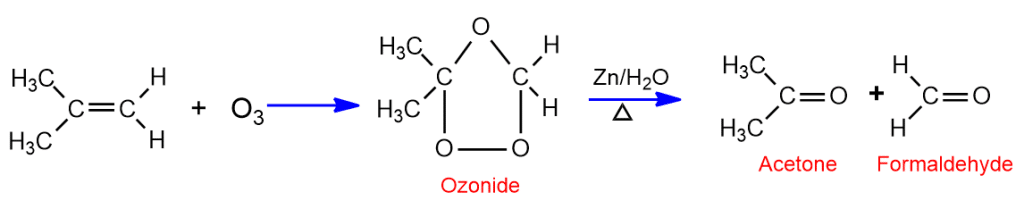
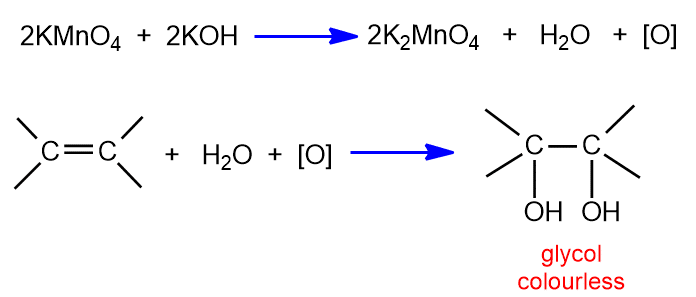
Oxidation with alkaline KMnO4 (Baeyer’s test)
Alkenes are readily oxidized by alkaline KMnO4 to glycol. This reaction is commonly known as Baeyer’s test for unsaturation.
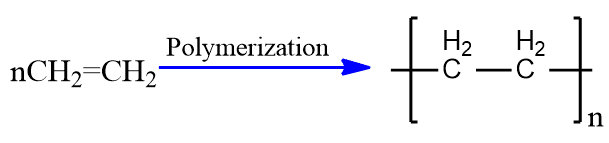
Polymerization
The process of making polymers from monomers is know as polymerization. The polymers are high molecular weight big molecules made by the polymerization of monomers. Generally organic peroxide (R-O-O-R), HF or H2SO4 and high temperature and pressure is required for the polymerization. Byty polymerization process large number of synthetic polymers are prepared. For example Polyethylene, Polypropylene etc.