Table of Contents
ToggleChichibabin Pyridine synthesis was first of all reported by Chichibabin in 1906. The general reaction, proposed mechanism, and its application have been discussed below.
Chichibabin pyridine synthesis reaction
It is the thermal cyclo-condensation of aldehydes and ammonia over a contact catalyst such as alumina to produce substituted pyridines. This reaction is called chichibabin pyridines synthesis or chichibabin synthesis. It is also known as the chichibabin Bayer reaction or the chichibabin cyclotrimerizaiton.
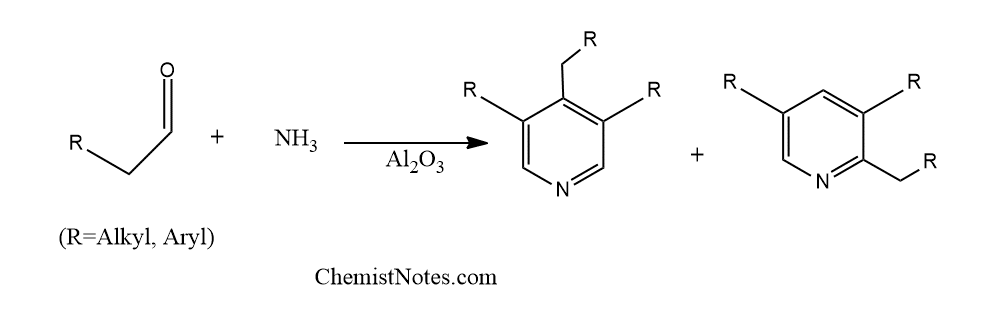
Chichibabin pyridine synthesis also takes place using aliphatic and aromatic ketones, alpha, beta-unsaturated aldehydes, and keto acids. However, when higher aliphatic aldehydes react with ammonia at high temperatures, anomalous pyridine derivatives occur in addition to the predicted pyridines.
Chichibabin pyridine synthesis reaction mechanism
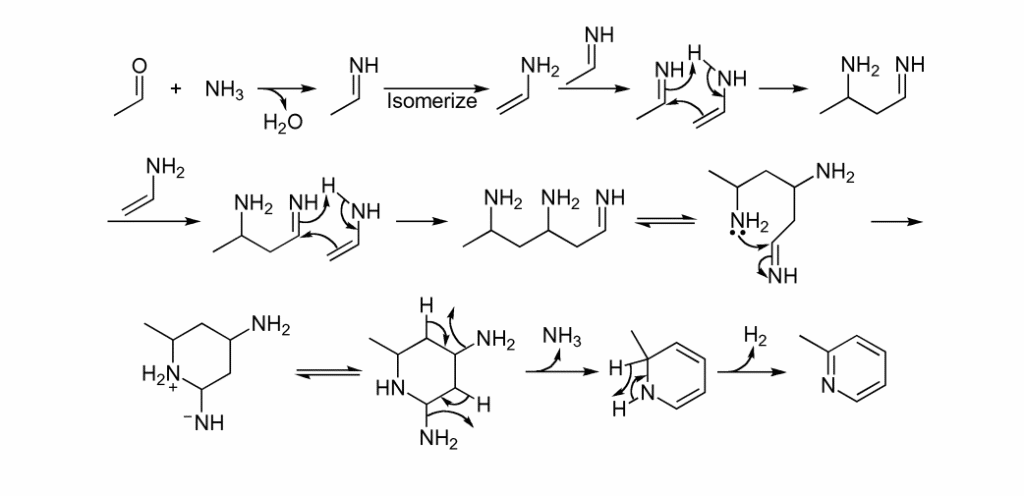
This reaction is thought to happen through a sequence of aldol condensations, followed by cyclization and dehydrogenation. The proposed mechanism of this synthesis is shown below:
Application of Chichibabin pyridine synthesis
This process is used to make pyridines and collidine derivatives.






