Table of Contents
ToggleWhat is Ester hydrolysis?
Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. Ester hydrolysis is usually catalyzed by acid or bases.
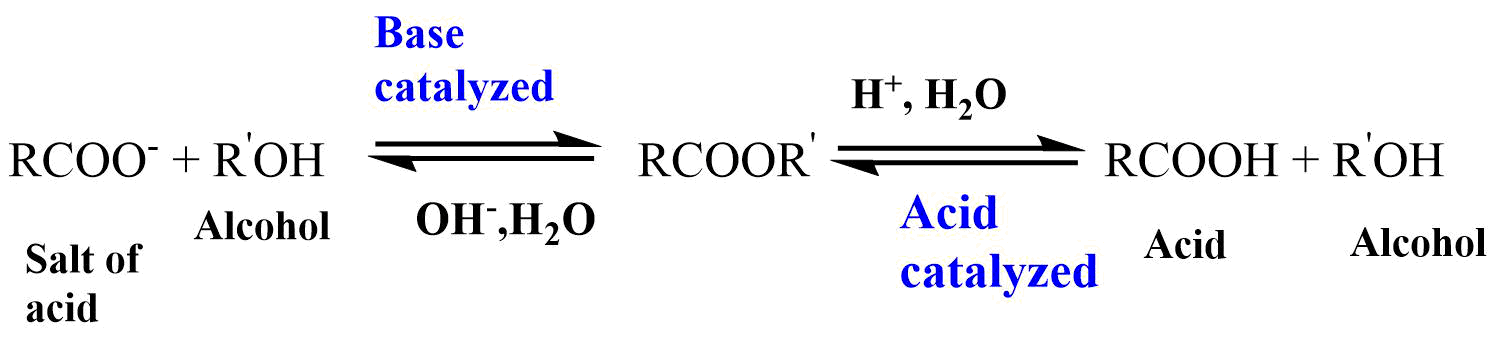
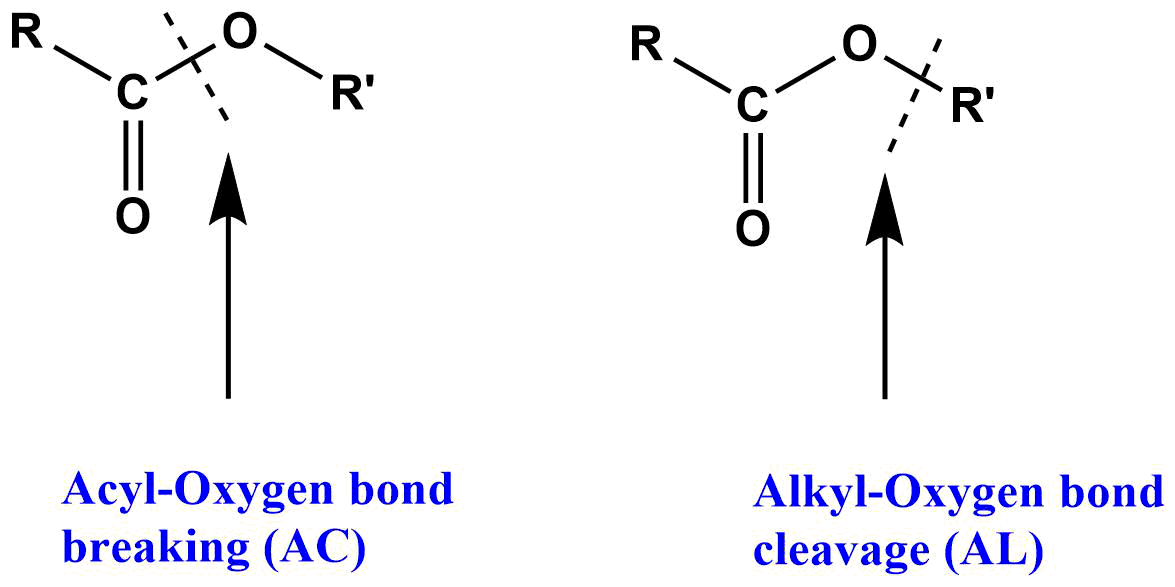
The hydrolysis of ester may involve acyl-oxygen bond breaking (more common) or alkyl-oxygen bond breaking (less common).
Since, -OR’ (alkoxy) is a much poorer leaving group than halide or -OCOR (carboxylate) group, water alone does not hydrolyze much esters. So, acid or base is needed for the hydrolysis of esters. When bases catalyze the reaction, the attacking species is more powerful nucleophile OH–. This reaction is called saponification and gives the salt of acids.
Acids catalyze the reaction by making the carbonyl carbon more positive, and therefore more susceptible to attack by the nucleophile. Both the reactions are reversible reactions, so, there must be a way to shift the equilibrium to the right for the reaction to be useful.
Classification of ester hydrolysis
Ingold has classified the acid and base catalyzed hydrolyses of esters (and the formation of esters, since these are reversible reactions and thus have the same mechanisms) into eight possible mechanisms depending on the following criteria:
- acid or base catalyzed
- unimolecular or bimolecular type, and
- acyl bond cleavage or alkoxy bond cleavage.
All of these eight mechanisms proceed through either SN1, SN2, or tetrahedral mechanism. The acid catalyzed mechanisms are shown with reversible arrows. They are not only reversible, but also symmetrical; that is, the mechanisms for ester formation are exactly the same as for hydrolysis, except that H is replaces R. Internal proton transfer may not actually be direct but may take place through the solvent.
Eight mechanisms of Ester hydrolysis
The designation AAC1, AAC2, AAL1, and AAL2 are used for acid catalyzed mechanism where A represent the acid catalyzed AC represent the acyl bond cleavage, AL represent the alkoxy bond cleavage and 1 & 2 represent the unimolecular and bimolecular type mechanism respectively. Similarly, base catalyzed reaction is represented by BAC1, BAC2, BAL1, and BAL2, where B represent the base catalyzed, AC represent the acyl bond cleavage, AL represents the alkoxy bond cleavage and 1 & 2 represent the unimolecular and bimolecular reaction respectively.
On the basis of the above three features, eight possible mechanisms of the ester hydrolysis can be imagined and among them six mechanisms have been observed.
| Types | Remarks |
| BAC2 | Very common, includes almost all basic ester hydrolysis (Saponification). |
| AAC2 | Very common, includes acid hydrolysis of esters of 1o and 2o alcohols. |
| BAC1 | Not observed till now. |
| AAC1 | Rare; occurs in concentrated solutions of very strong acids. |
| BAL2 | Extremely rare; only for β-lactones in absence of strong acids and bases. |
| AAL2 | Not observed till now. |
| BAL1 | Common for esters of 3o alcohol and also for esters of alcohol that can form stable carbocations. Not observed in concentrated bases. |
| AAL1 | Common for esters of 3o alcohol and also for esters of alcohol that can form stable carbocations. Not observed in concentrated bases. |
The eight possible mechanism of ester hydrolysis are as shown below:
1) AAC1 (Acid catalyzed acyl bond cleavage unimolecular reaction)
It is unimolecular reaction involving acyl-oxygen bond cleavage where nucleophilic attack occurs on the conjugate acid of esters in acidic condition. The reaction proceeds via SN1 type mechanism.
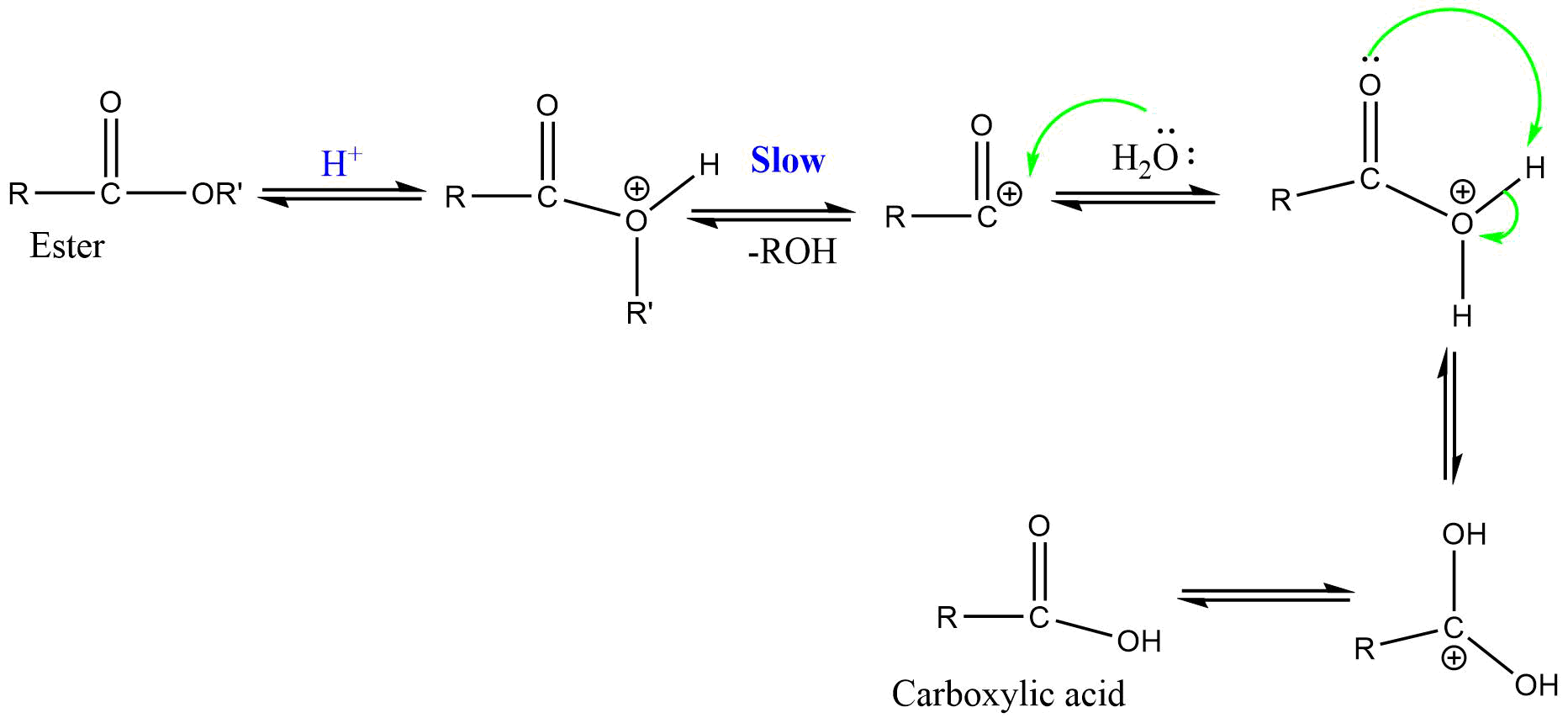
2) AAC2 (Acid catalyzed acyl bond cleavage bimolecular reaction)
It is a bimolecular reaction involving acyl-oxygen bond cleavage where nucleophile attacks on the conjugate acid of ester in acidic condition. This reaction proceeds via tetrahedral mechanism.
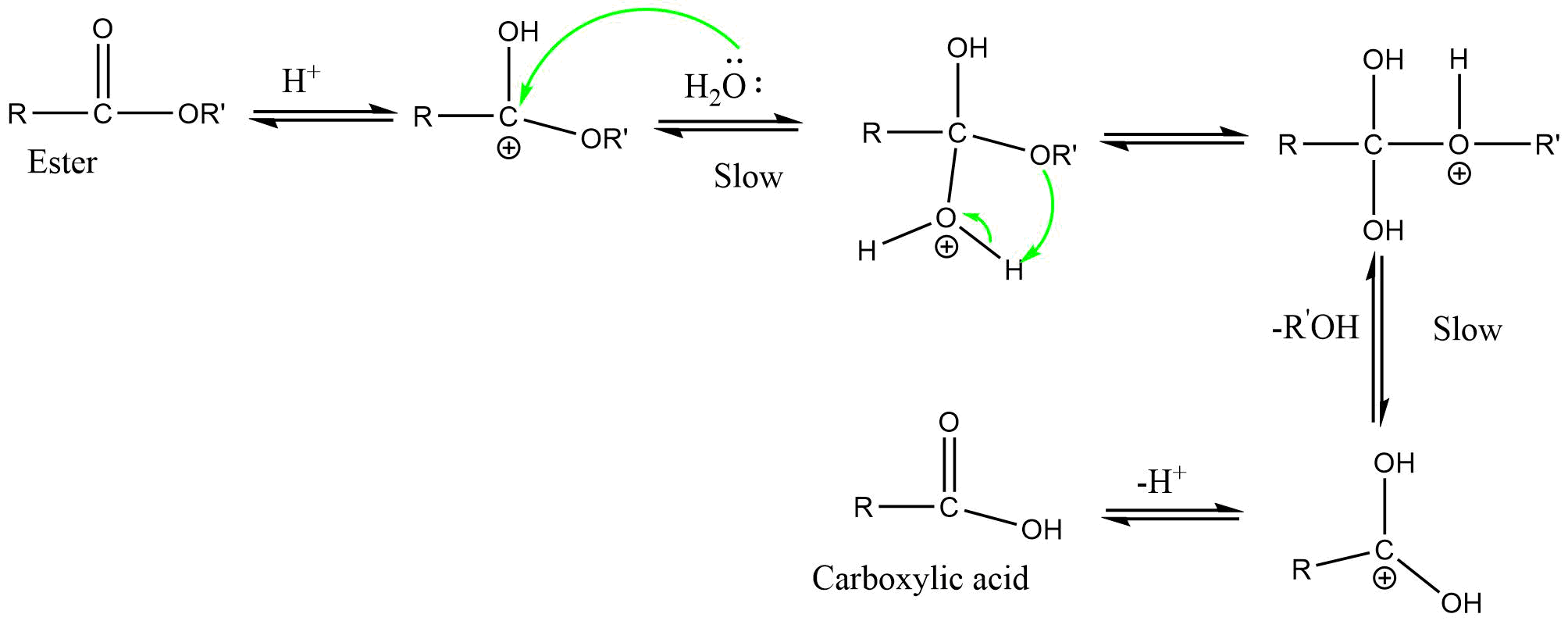
3) AAL1 (Acid catalyzed alkyl-oxygen bond cleavage unimolecular reaction)
It is a unimolecular reaction involving alkyl-oxygen bond cleavage, where nucleophile attacks on the conjugate acid of ester in acidic condition. This reaction also proceeds through SN1 type mechanism.
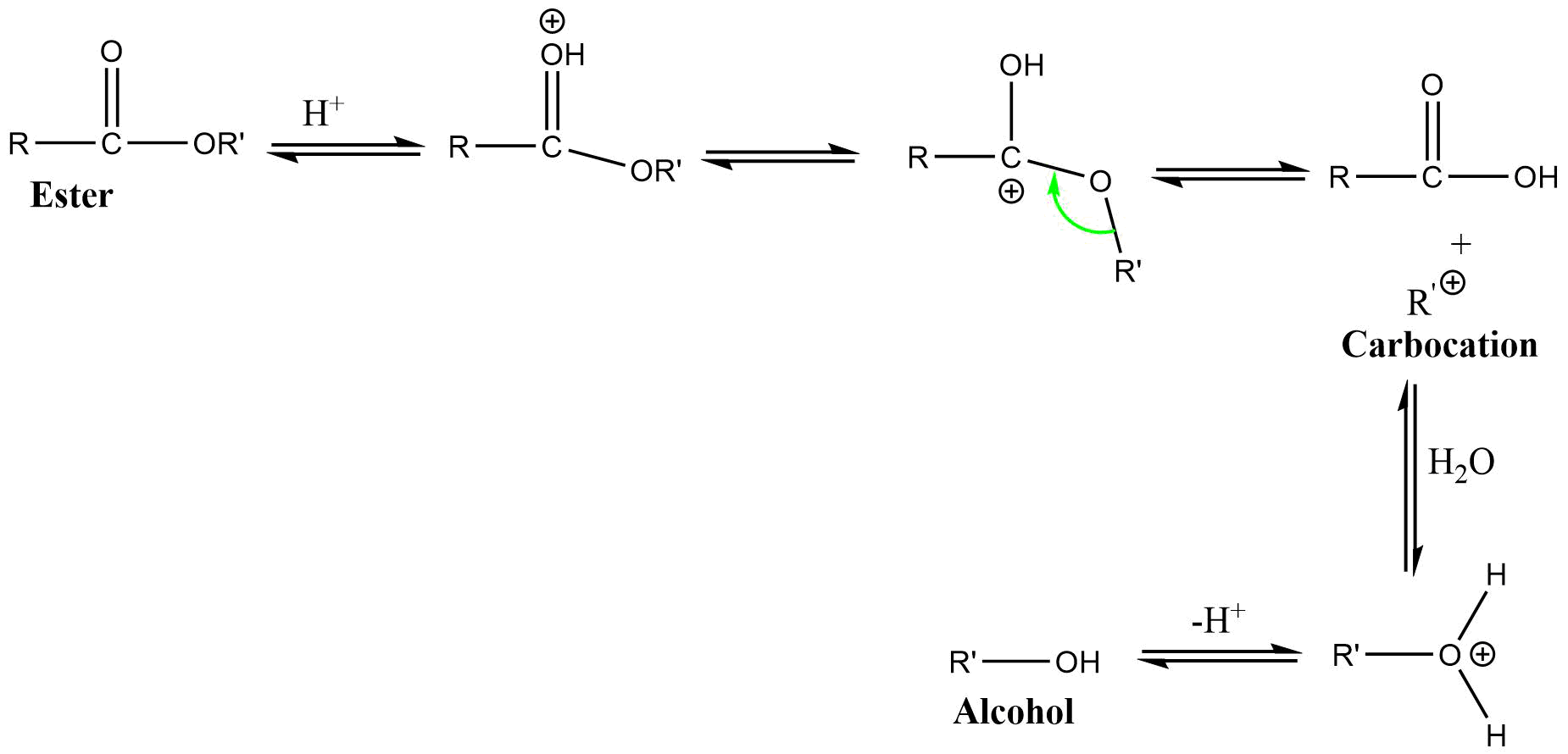
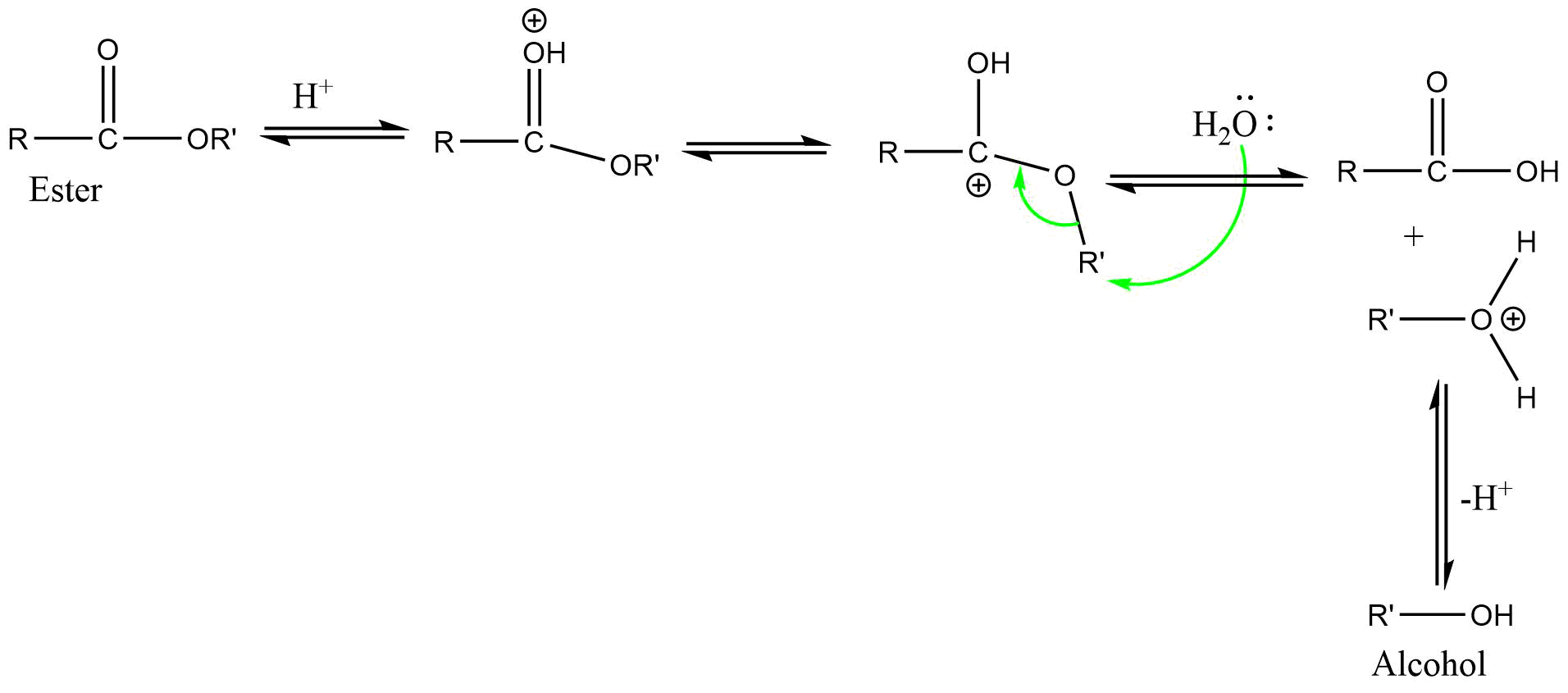
4) AAL2 (Acid catalyzed alkoxy bond cleavage bimolecular reaction)
It is a bimolecular reaction involving alkyl-oxygen bond cleavage, where nucleophilic attack occurs on the conjugate acid of esters in acidic condition. The reaction proceeds via SN2 type mechanism.
5) BAC1 (Base catalyzed acyl bond cleavage unimolecular reaction)
It is a unimolecular reaction involving acyl-oxygen bond cleavage, where nucleophile attacks on the ester itself in the base catalyzed or neutral condition. The reaction proceeds by SN1 mechanism.
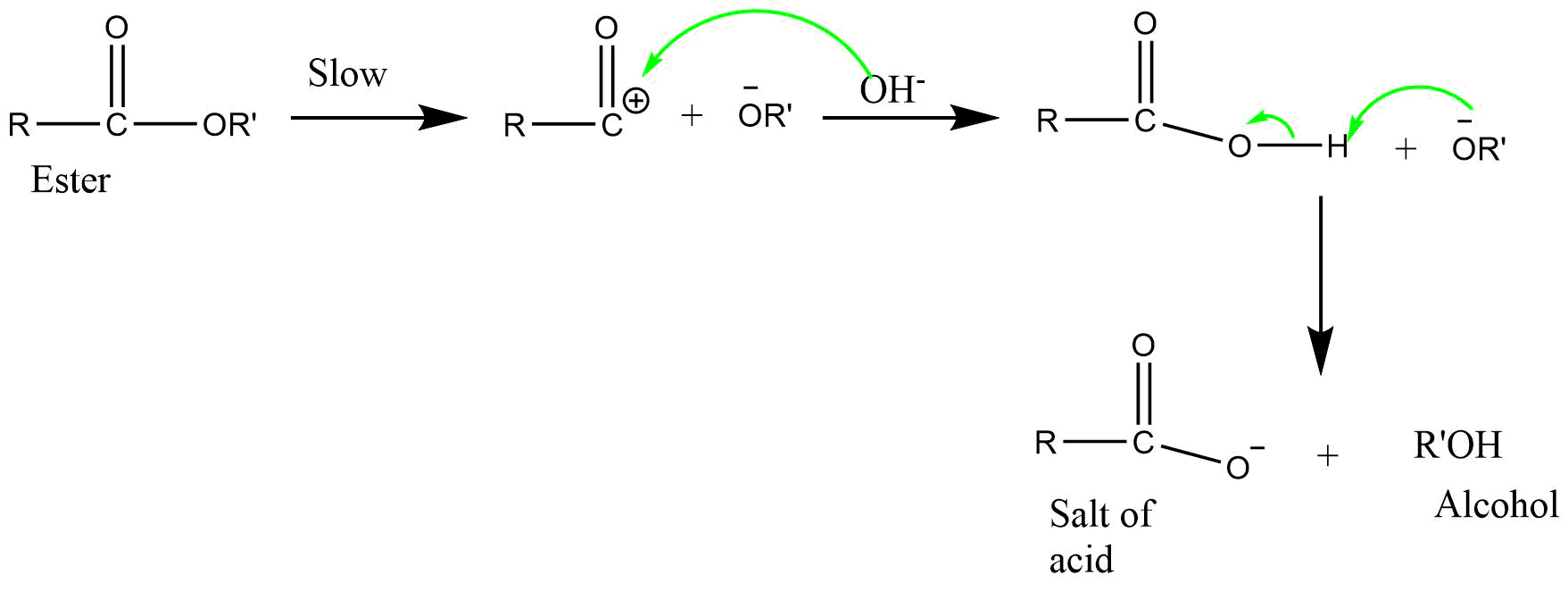
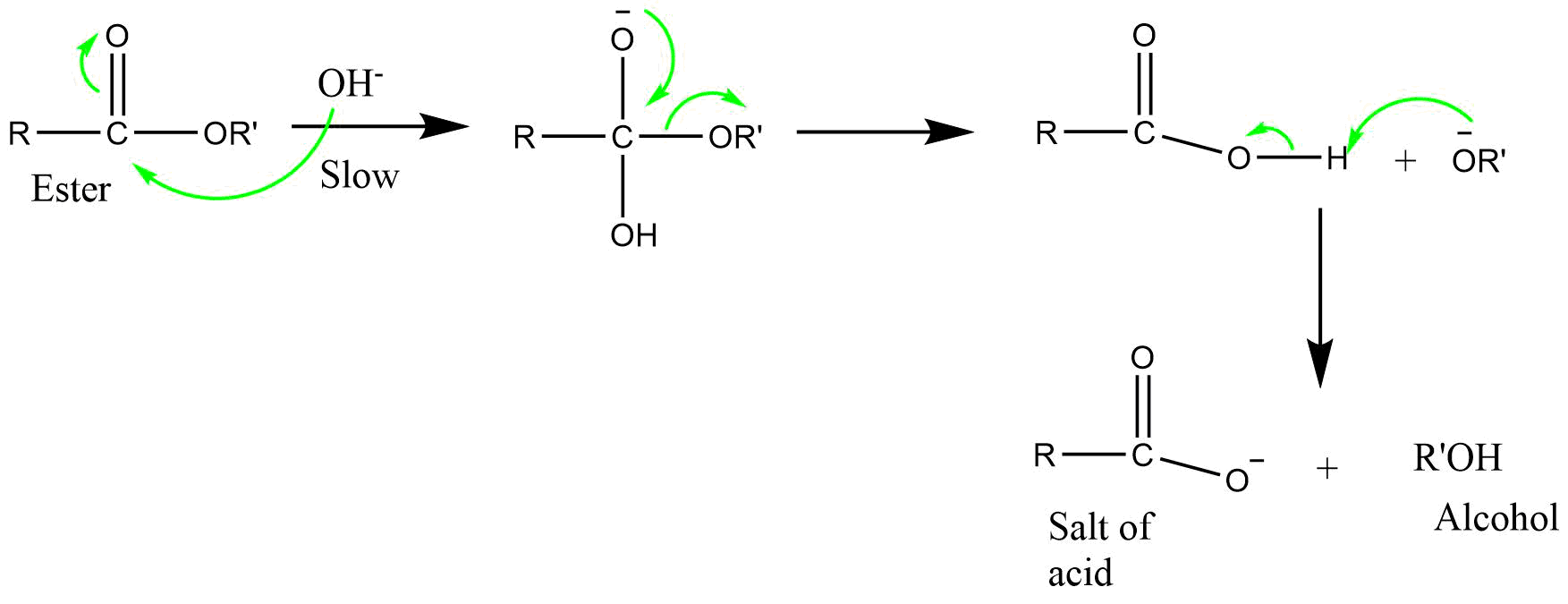
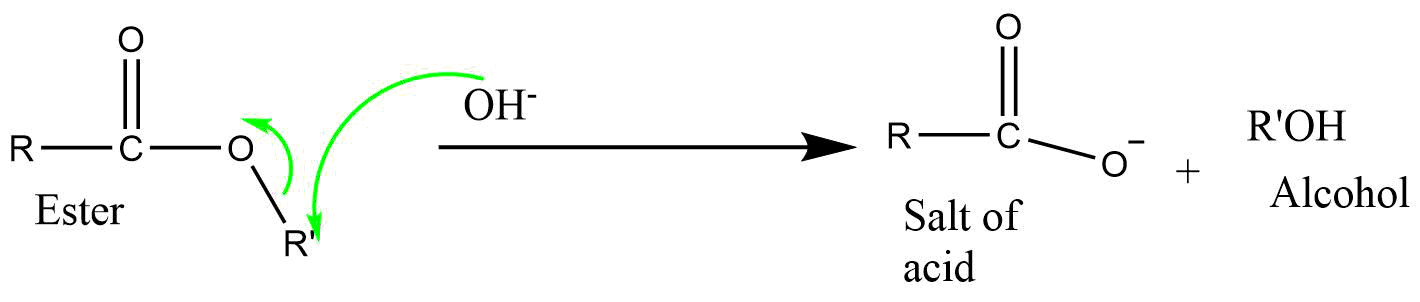
6) BAC2 (Base catalyzed acyl bond cleavage bimolecular reaction)
It is a bimolecular reaction involving acyl-oxygen bond cleavage, where nucleophilic attack occurs on the ester itself in the base catalyzed or neutral condition. The reaction proceeds via tetrahedral mechanism.
7) BAL1 (Base catalyzed alkoxy bond cleavage unimolecular reaction)
It is a unimolecular reaction involving alkyl-oxygen bond cleavage in which attack occurs on the ester itself in base catalyzed or neutral condition. The reaction proceeds via SN1 type mechanism.
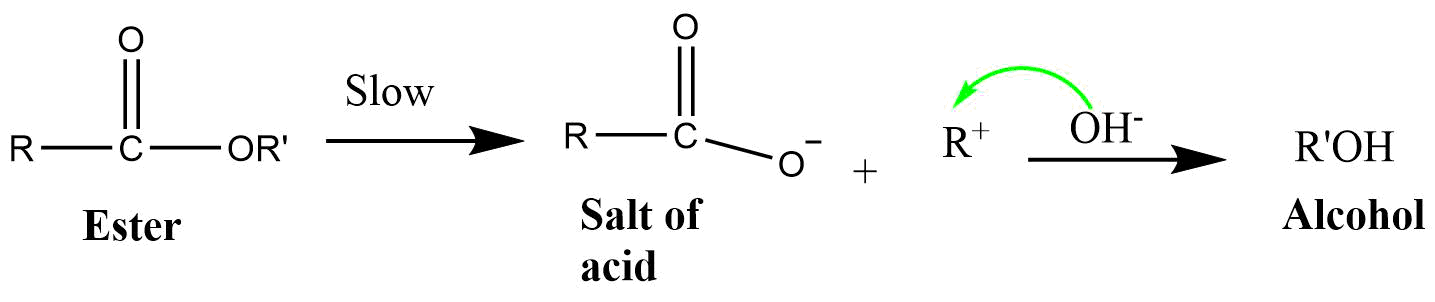
8) BAL2 (Base catalyzed alkoxy bond cleavage bimolecular reaction)
It is a bimolecular reaction involving alkyl-oxygen bond cleavage in which nucleophilic attack occurs on the ester itself in base catalyzed or neutral condition. The reaction proceeds via SN2 mechanism.
Ester hydrolysis video
Published by: Siddha Raj Upadhyaya
References:
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992
- https://en.wikipedia.org/wiki/Christopher_Kelk_Ingold






