Table of Contents
ToggleBenzyne or arynes are highly reactive reaction intermediates that are derived formally by the removal of two adjacent substituents from aromatic rings, leaving behind two electrons to be distributed between two orbitals.
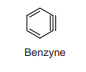
Benzyne lewis structure
Benzyne is usually represented as a single molecule with a carbon-carbon triple bond. This strained π-bond is formed by the lateral overlap of the two orbitals in the plane of the ring. In fact, one π bond is normal and is just part of the aromatic system. The new π bond formed by the overlap of two sp2 orbitals outside the ring is abnormal and quite strained. This external π bond is very weak, which is why benzyne is a very unstable and highly reactive intermediate.
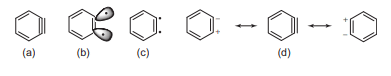
Benzyne precursor
The precursor to benzyne is benzene diazonium-2-carboxylate which decomposes to give benzyne as intermediate.
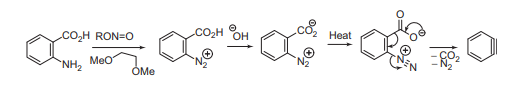
Benzyne formation mechanism/Generation of Benzyne intermediate
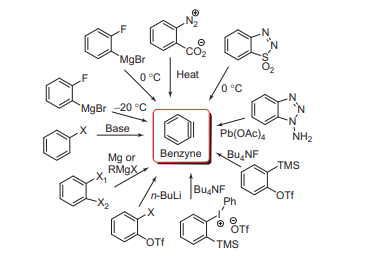
Some of the common methods of Benznye formation are shown below in the figure.
Bromobenzene is treated with a strong base like sodium amide to generate benzyne intermediate, which reacts with ammonia solution to give aniline.
Mechanism
- Formation of benzyne intermediate: Base abstracts a proton from ortho position to form carbanion which losses bromine atom to generate benzyne intermediate.
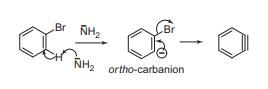
2. Formation of the final product:
Benzyne reacts with ammonia to form aniline. There may occur either ipso or cine substitution.
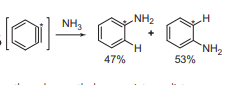
Benzyne properties
- Benzyne shows the properties of a highly reactive alkyne, participating in a range of cycloaddition reactions.
- benzyne resembles a carbene, having a similar electronic arrangement of two electrons distributed between two orbitals and behaving as a powerful electrophile.
- Benzyne is electron-deficient and will be attacked by nucleophiles in a reaction that opens the π bond not part of the aromatic cloud, and produces a new carbanion. Protonation completes the sequence to give the aromatic substitution product.
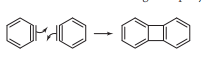
Benzyne dimerization
The dimerization of benzyne intermediate takes place as shown in the following figure.
FAQs:
what is benzyne?
Benzyne is a very reactive intermediate species that are derived formally by the removal of two adjacent substituents from aromatic rings, leaving behind two electrons to be distributed between two orbitals.
why is benzyne so reactive?
The new π bond in benzyne is formed by the overlap of two sp2 orbitals outside the ring is abnormal and quite strained. This external π bond is very weak, that is why benzyne is a very unstable and highly reactive intermediate.






