Table of Contents
ToggleLaboratory Preparation of nitrobenzene from benzene, purification, and its uses in organic chemistry have been discussed here:
Principle involved in laboratory preparation of Nitrobenzene
In the laboratory, nitrobenzene is prepared by heating benzene with a mixture of Conc. HNO3 and Conc. H2SO4 at 600C. It is an electrophilic substitution reaction as it takes place by the attack of electrophilic reagent nitronium ion NO2+ at the position of high electron density in the ring.
The reaction involving preparation of nitrobenzene by nitration of benzene can be represented as:
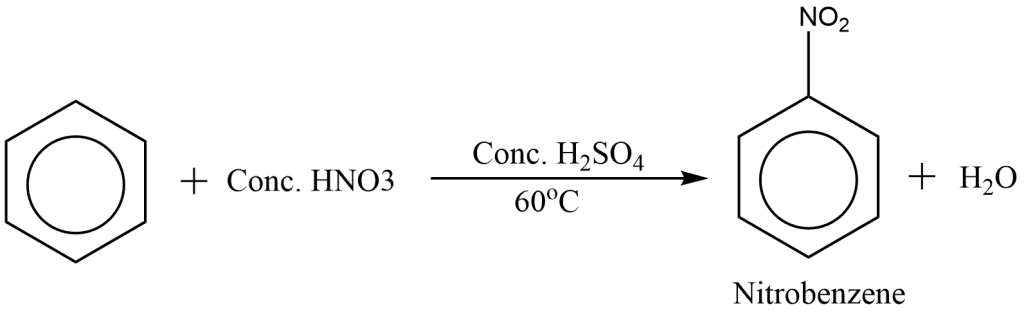
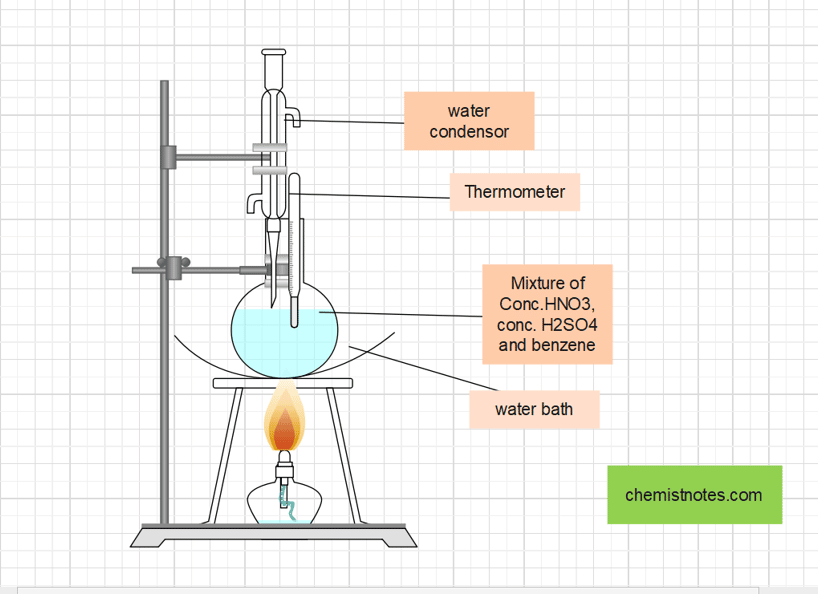
Procedure for the laboratory preparation of Nitrobenzene
A mixture of 35 mL of Conc. HNO3 and 40 mL of Conc.H2SO4 are taken in a round bottom flask. To this flask, 30 mL of benzene is added with constant shaking. Then the mixture is heated (refluxed) in the water bath at 60oC for about one hour till the yellow oily layer of nitrobenzene having a bitter almond odor appears on the surface. The flask is then cooled, and the nitrobenzene layer is separated using a separating funnel.
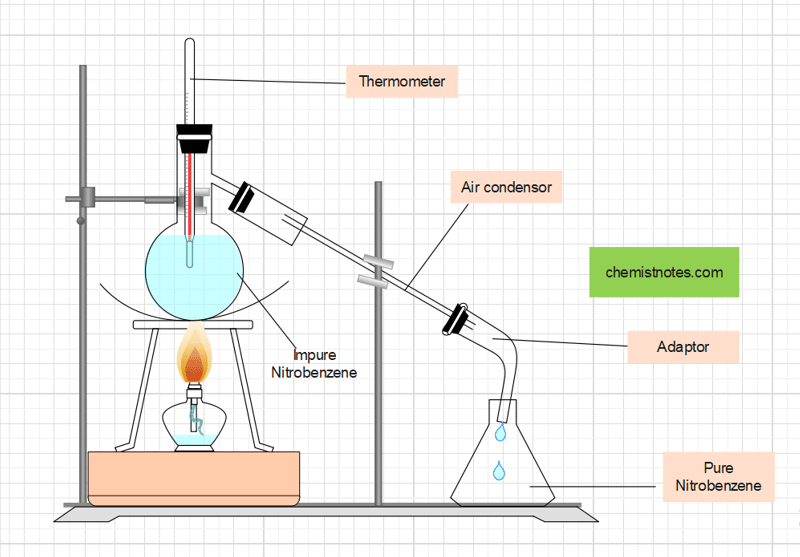
Purification of Nitrobenzene
The impure nitrobenzene is contaminated with acid impurities. Thus, to neutralize the excess of acid, it is first treated with Na2CO3, and then with water several times. Now, It is dried with anhydrous calcium chloride (CaCl2) to remove water content, and then finally distilled at about 2110C using an air condenser to get pure and dry nitrobenzene.
Uses of Nitrobenzene
- For the preparation of aniline
- For the manifacture of shoe polishes, floor polishes, and so on.
- As an oxidizing agent in organic symthesis.
- Also used for scenting cheap soaps.
Preparation of nitrobenzene video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.






