Table of Contents
ToggleLaboratory Preparation of aniline, chemical reactions, and its uses in organic chemistry have been discussed here:
Principle involved in laboratory preparation of Aniline
Aniline is prepared in the laboratory by the reduction of nitrobenzene with tin (Sn) and Concentrated hydrochloric acid (HCl).
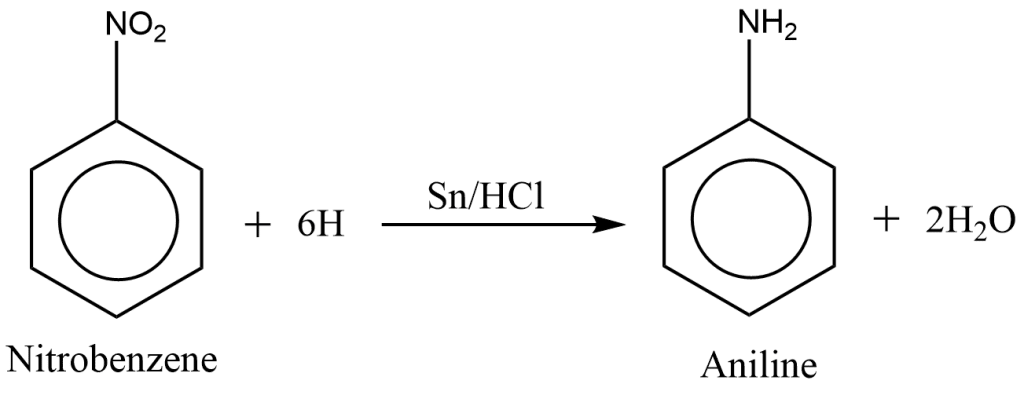
The following reactions are involved in the preparation of aniline.
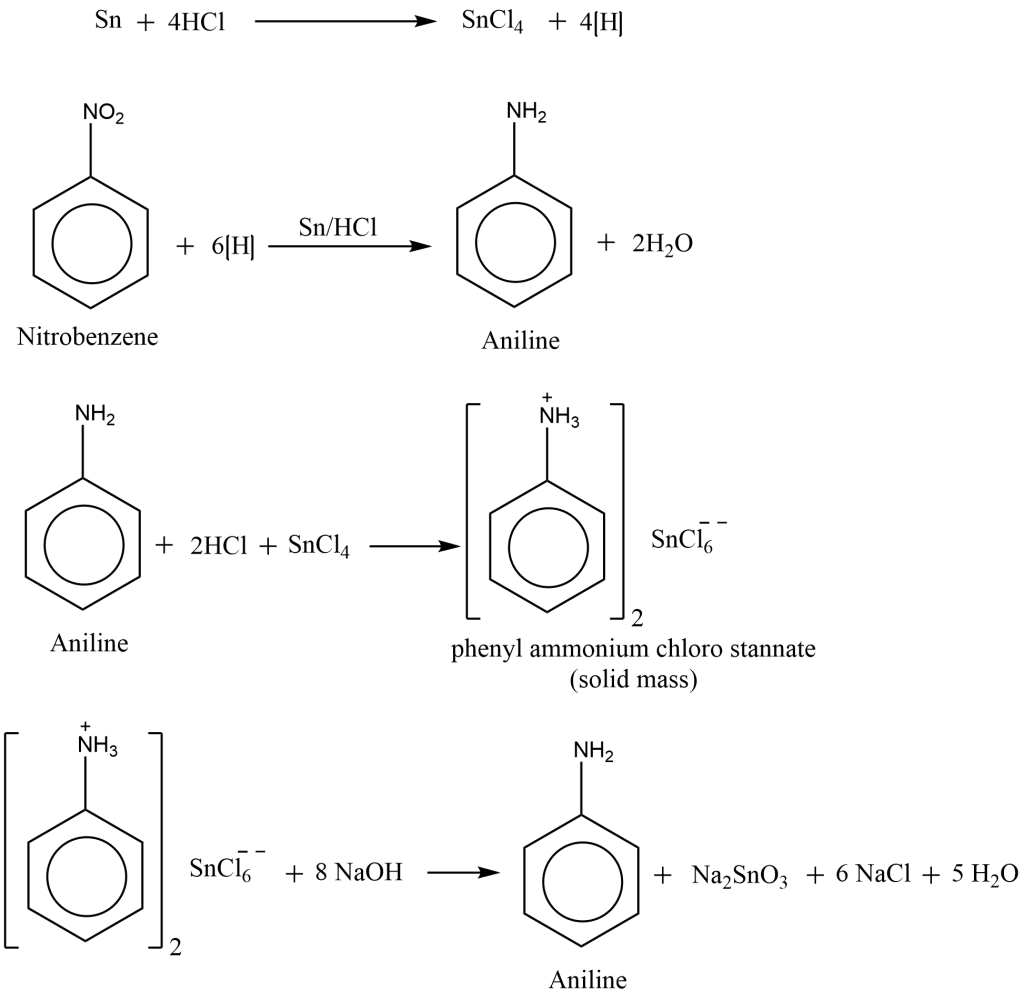
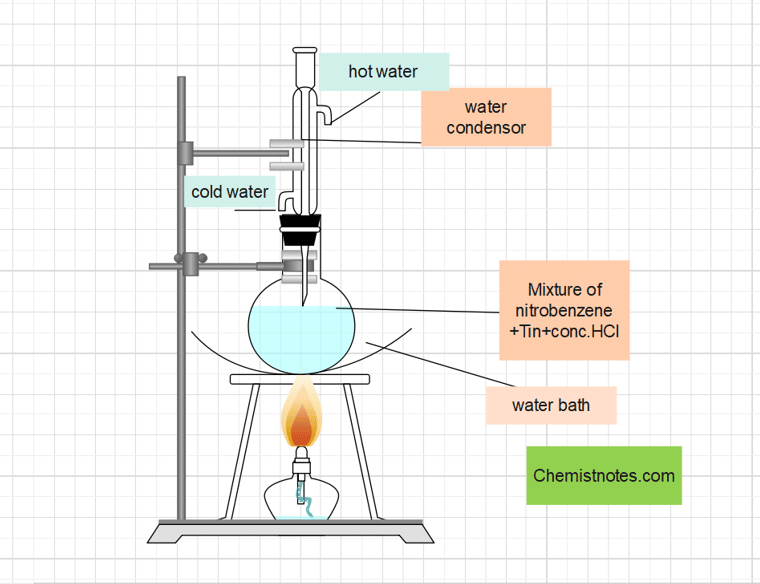
Procedure for the laboratory preparation of Aniline
In a 250 mL round bottom flask fitted with a reflux condenser, 20 mL nitrobenzene and 40 gm granulated tin are added. 50 mL of Conc. HCl is added slowly with constant shaking. The round bottom flask is cooled after each addition so that the temperature does not exceed 60°C. The reaction mixture is then heated for approximately an hour in a boiling water bath until the oily droplets of nitrobenzene disappeared. After cooling the flask, a crystalline solid mass known as phenyl ammonium chloro stannate is separated. The impure alkaline as a dark brown oil is then obtained by treating the crystalline solid mass with Conc. NaOH.
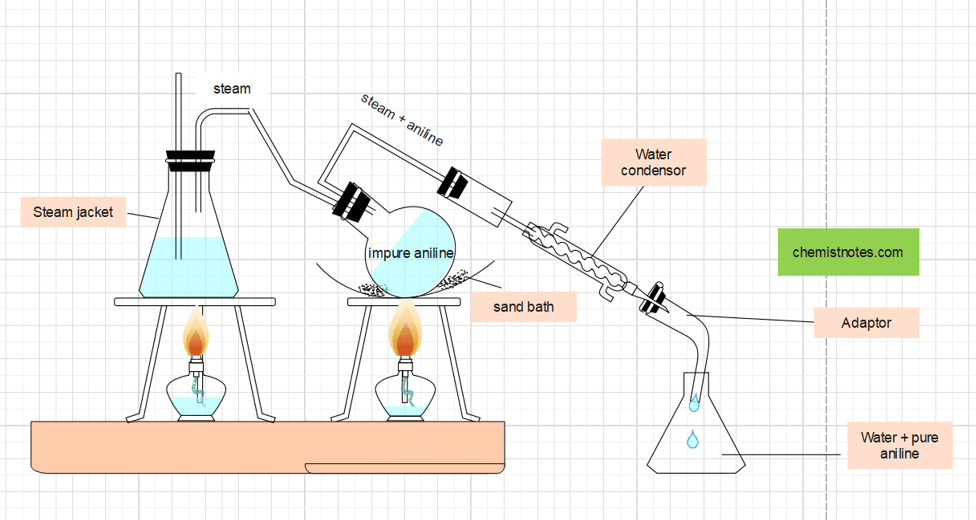
Purification of Aniline
The mixture obtained is then subjected to the process of steam distillation to separate aniline. Distillate that contains a mixture of aniline and water is extracted several times with ether. Aniline thus obtained is redistilled at 182-1850C to obtain pure and dry aniline.
Chemical Reactions of Aniline
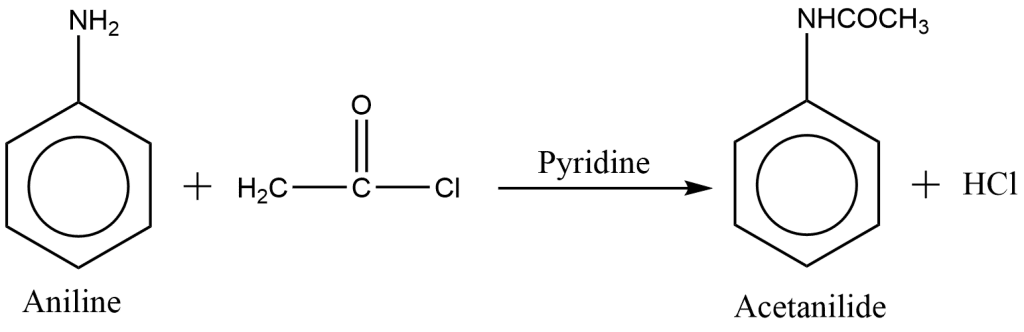
preparation of acetanilide from aniline reaction
In reaction with an acid chloride or acid anhydride, aniline gives N-acyl product in presence of a catalyst.
preparation of 2, 4, 6-tribromoaniline from aniline
Aniline reacts with aqueous Br2 to give a white precipitate of 2,4,6-tribromoaniline.

preparation of diazonium salt from aniline
In reaction with nitrous acid (NaNO2 + HCl) in cold conditions, aniline gives benzene diazonium salt.
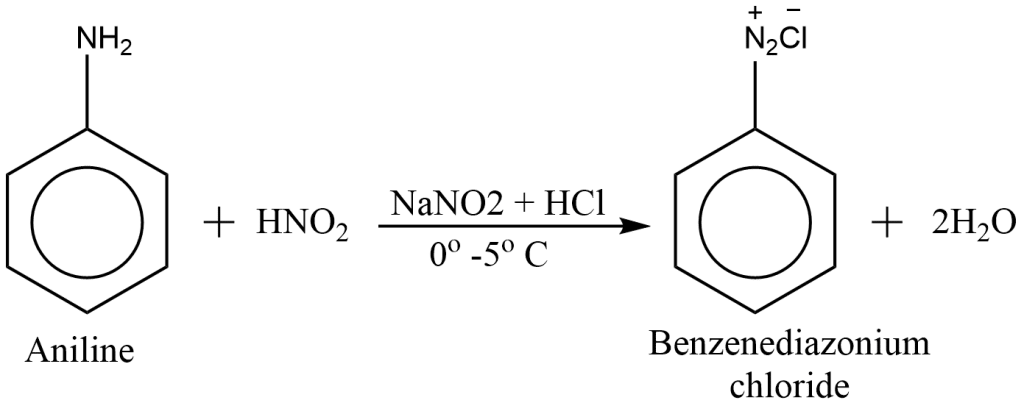
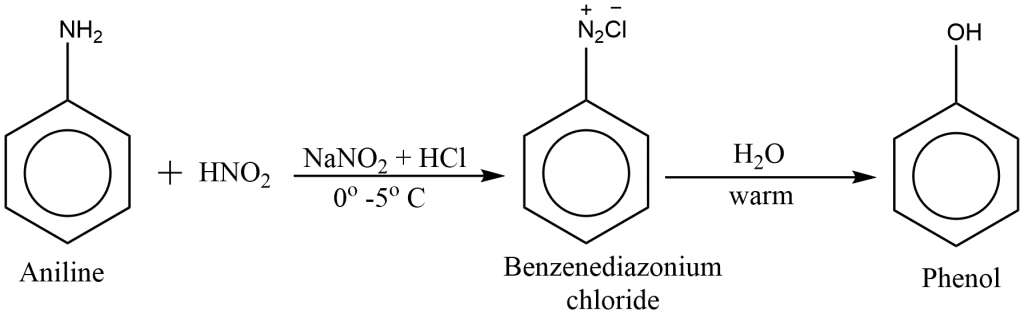
preparation of phenol from aniline
Benzene diazonium salt formed from the reaction of aniline with nitrous acid (NaNO2 + HCl) in cold conditions when is hydrolyzed, then phenol is obtained.
preparation of sulfanilic acid from aniline
When aniline is heated with fuming sulphuric acid, sulfanilic acid, or p-aminobenzenesulphonic acid is obtained.

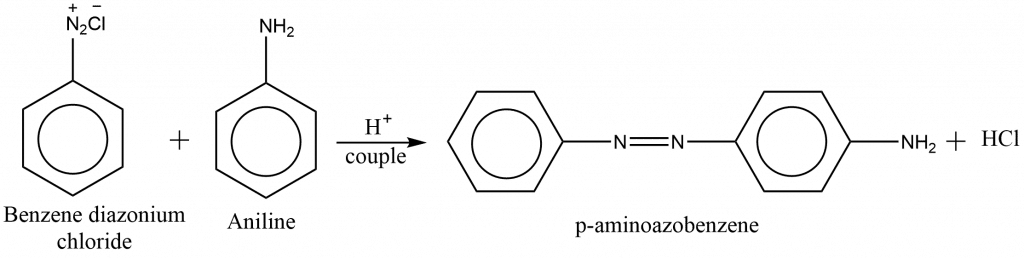
preparation of azo dye from aniline
Aniline when coupled with a benzene diazonium salt, it gives p-aminobenzene (orange-red dye).
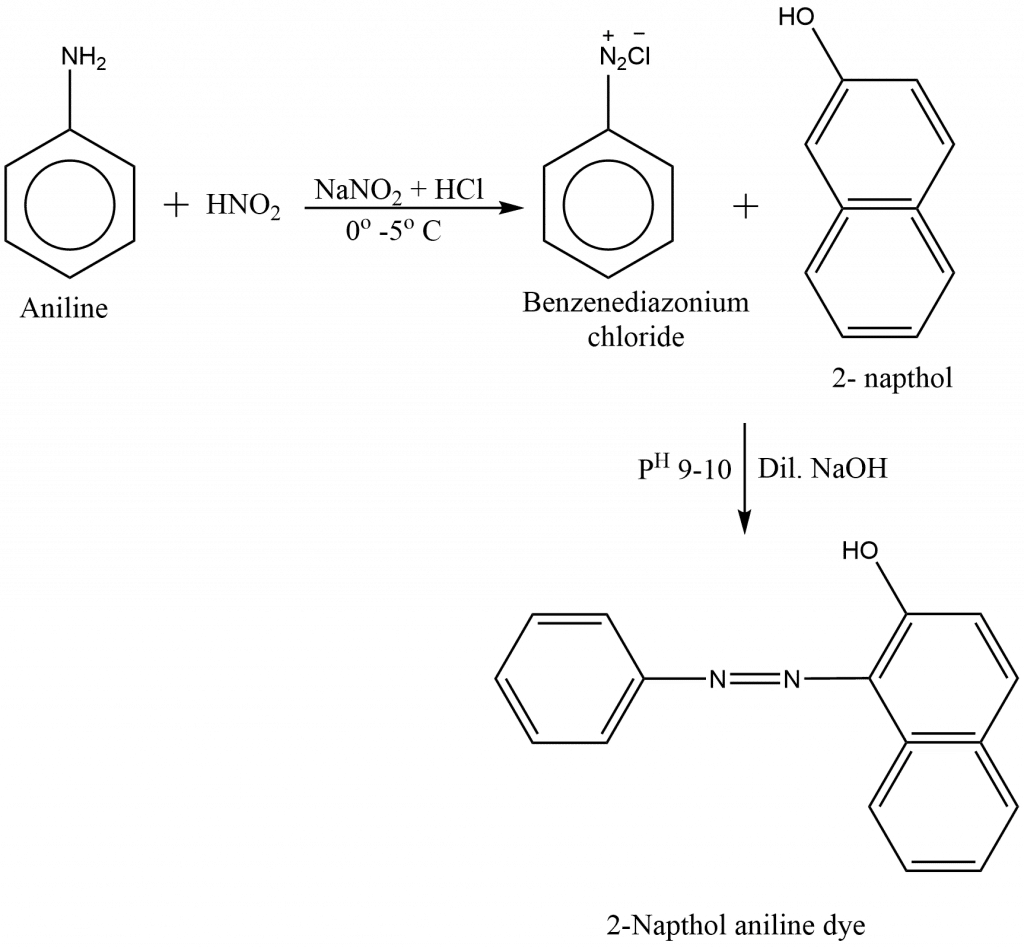
preparation of 2-naphthol aniline dye
Benzene diazonium salt when treated with 2-naphthol in presence of dilute NaOH at pH 9-10 yields 2-naphthol aniline.
preparation of aniline from nitrobenzene
Aniline is prepared when nitrobenzene is reduced with tin (Sn) and Concentrated hydrochloric acid (HCl).
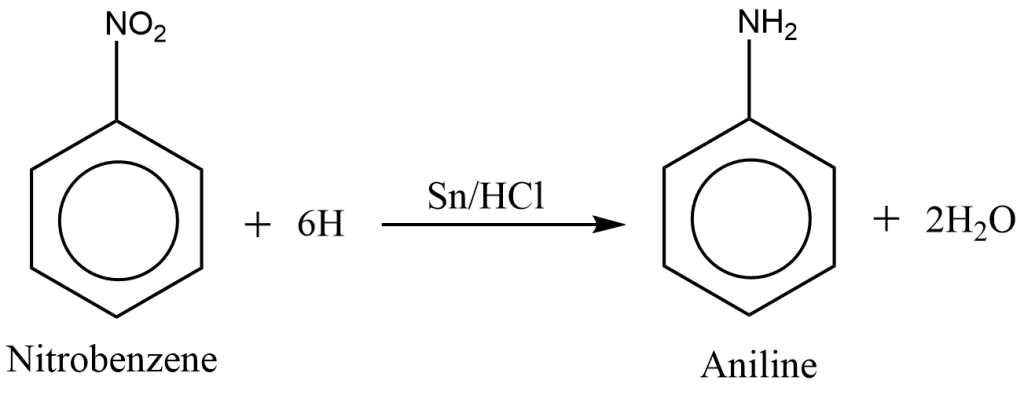
Uses of Aniline
- Uses as a pesticide and fungicides in the agricultural industry.
- For the manufacture of dyes, sulpha drugs etc.
- Used to determine the aromaticity of oil products.
- For the manufacture of antioxidants and vulcanization accelerators in rubber and plastics industry.
Preparation of Aniline video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.






