Table of Contents
ToggleKeto-enol tautomerism is a fundamental concept that investigates proton migration between two constitutional isomers, the keto and enol tautomers. This is the very common form of tautomerism in between a carbonyl compound containing α-hydrogen and its enol form. The keto form of the compound is usually more thermodynamically stable and favored. In some cases, however, the enol form may be more stable.
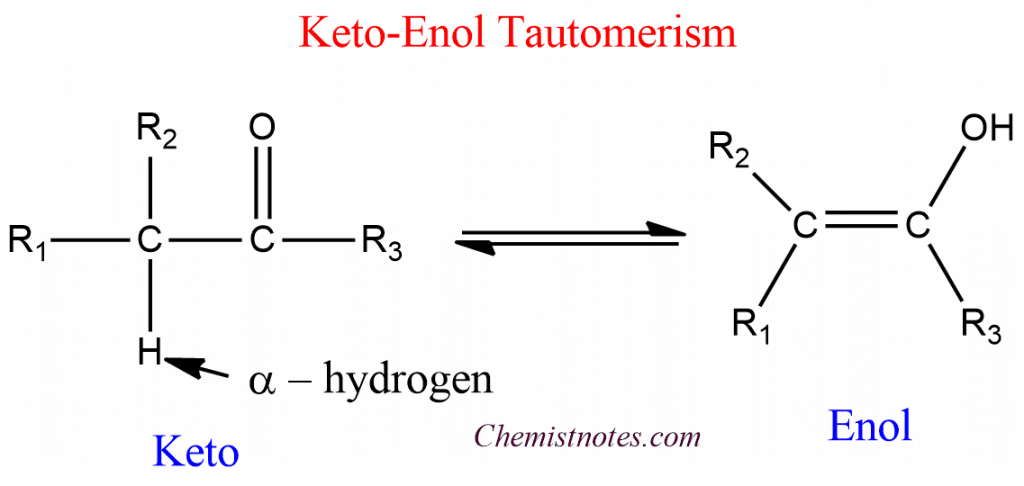
The keto form differs from the enol form by the presence of a C-H, C-C, and C=O bond, whereas the enol has a C=C, C-O, and O-H bond. The approximate sum of the three is 359 kcal mol-1 and of the second three is 347 kcal mol-1. The keto form is thermodynamically more stable by ~12kcal mol-1, and in most cases, the enol forms cannot usually be isolated.
There are three types of the more stable enols.
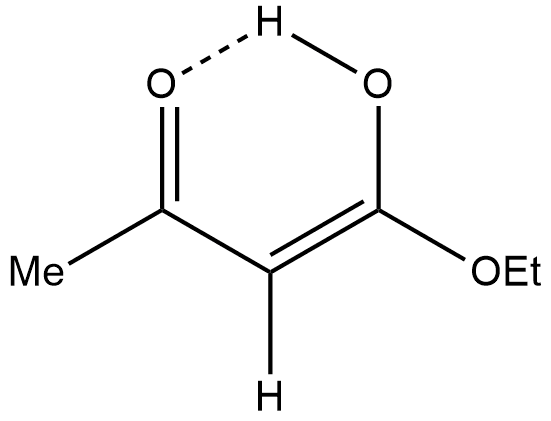
- The molecule in which the enolic double bond is in conjugation with another double bond. For example: In molecules like acetoacetic ester, the enol is also stabilized by internal hydrogen bonding, which is unavailable to the keto form.
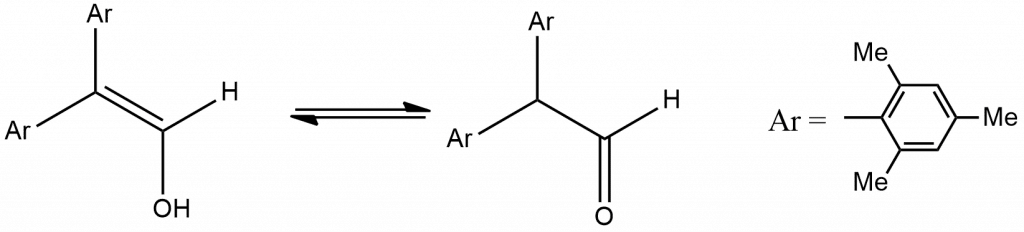
2. Molecules that contain two or three bulky aryl groups. For example An amide with a bulky aryl group [N-methyl bis(2,4,6-triisopropyl-phenyl)acetamide] that has a measurable enol content, in sharp contrast to most amides.
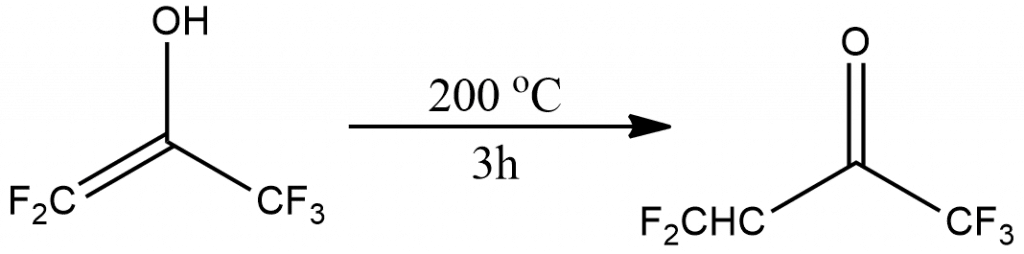
3. Highly fluorinated enols
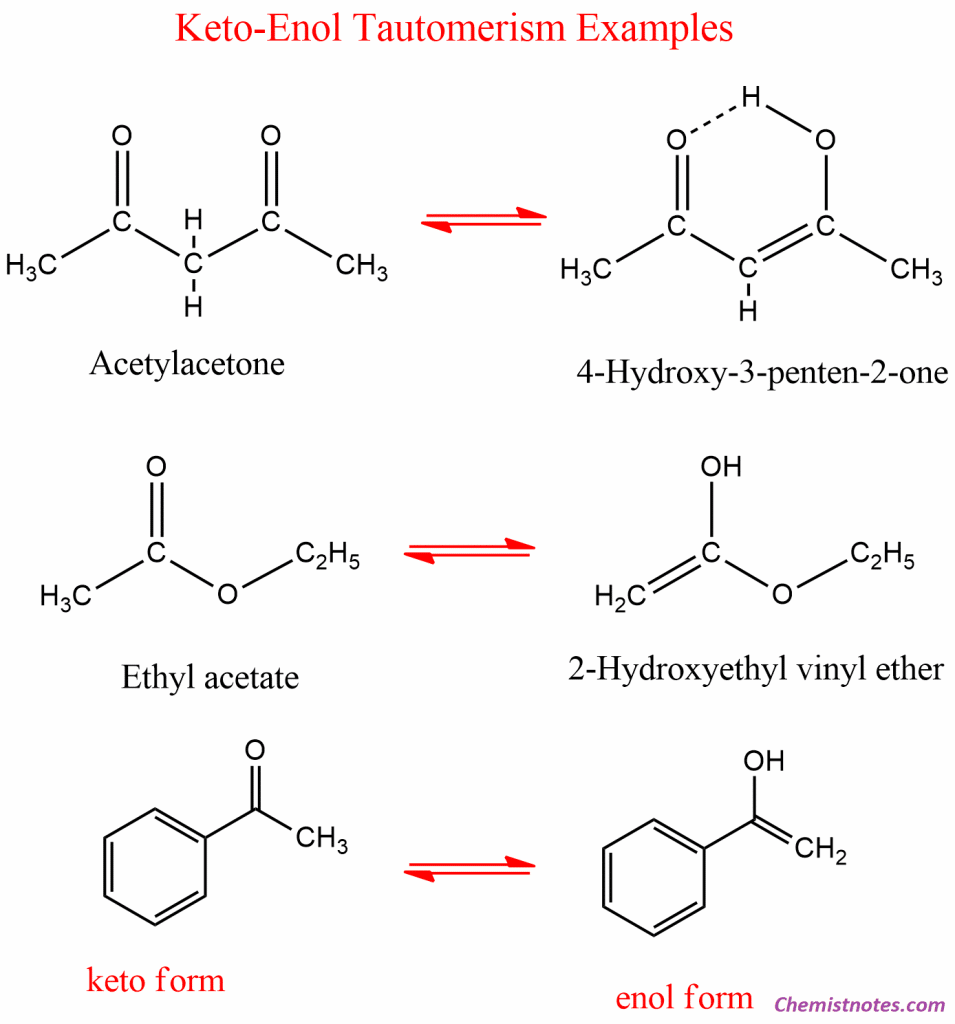
Examples of Keto-enol Tautomerism
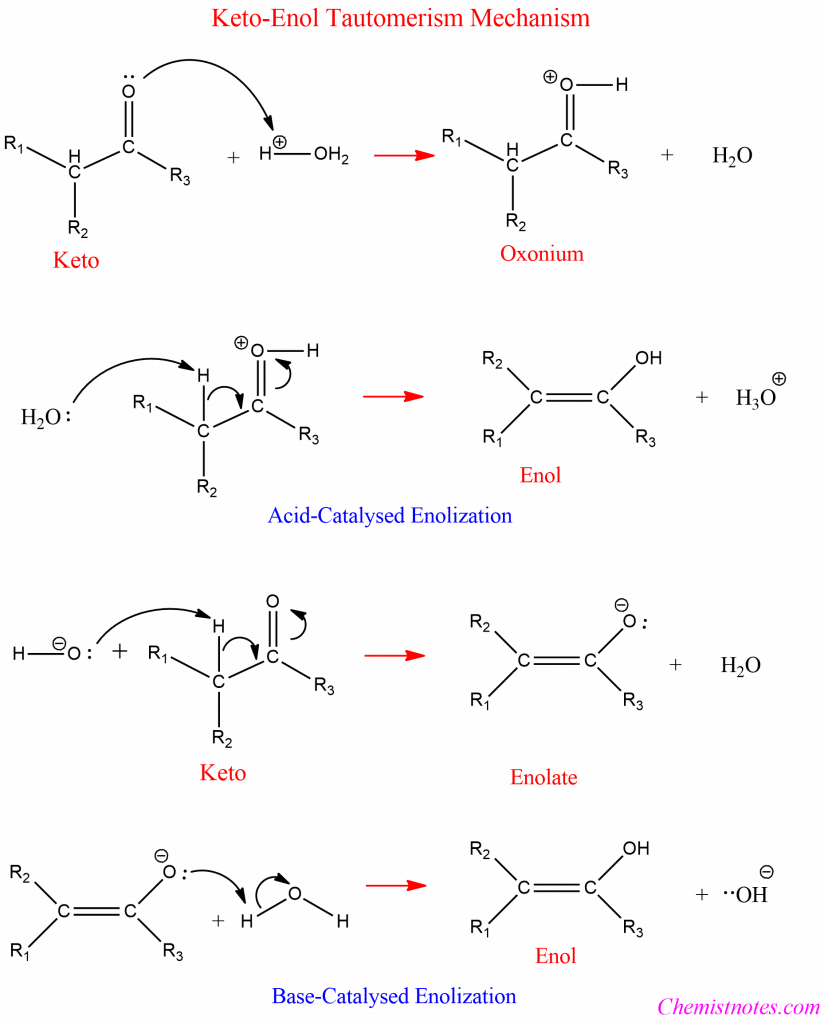
Mechanism of Keto-enol Tautomerism
Keto-enol tautomerism is the chemical equilibrium between a ketone or aldehyde and an enol. There are two distinct reaction pathways to be considered since the presence of either acidic or basic conditions leads to tautomerism. One applies to acidic conditions, while the other applies to basic conditions.
FAQs
What is keto-enol Tautomerism?
Keto-enol tautomerism is a fundamental concept that investigates proton migration between two constitutional isomers, the keto and enol tautomers.






