Table of Contents
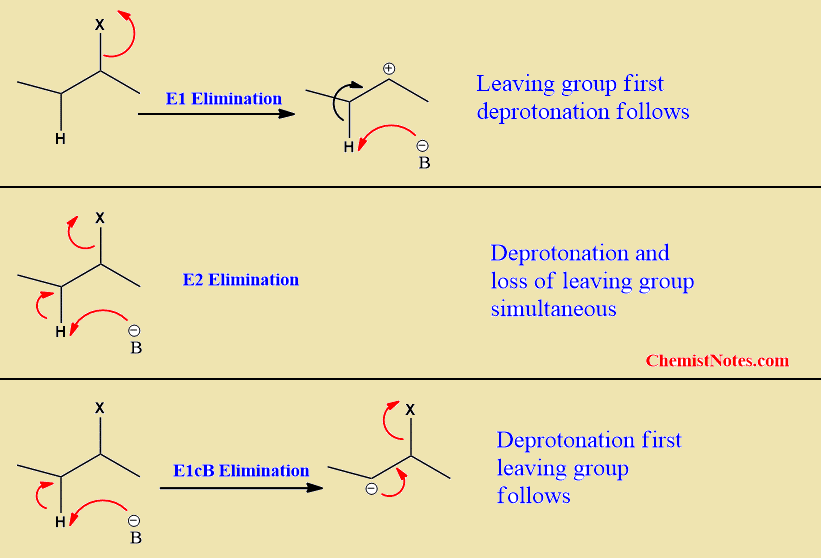
ToggleE1cB reaction is more common in those substrates having very poor leaving groups. The name E1cB comes from the fact that it is the conjugate base of the substrate that is giving up the leaving group.
E1cB reaction
In the E2 mechanism, hydrogen abstraction and the removal of leaving group are concerted processes. i.e these take place in a single step. In contrast, leaving group is removed at first to form a carbocation intermediate followed by the removal of the proton in the E1 mechanism.
But, in the E1cB mechanism, abstraction of the proton occurs first to give an intermediate carbanion which then gives an alkene upon departure of the leaving group(nucleofuge). This is a two-step mechanism. Here, ‘cB’ stands for ‘conjugate base’ since the carbanion intermediate is the conjugate base of the substrate, and the departure of leaving group from the conjugate base is unimolecular. Thus, it is termed E1cB.
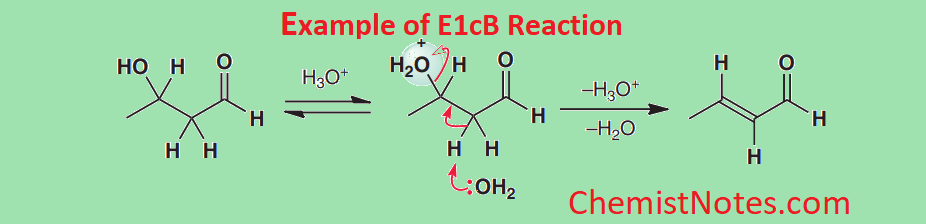
E1cB reaction examples
The E1cB mechanism is not very common, but the base-catalyzed dehydration step in the aldol reaction is a typical and important example. Many other reactions are also known.
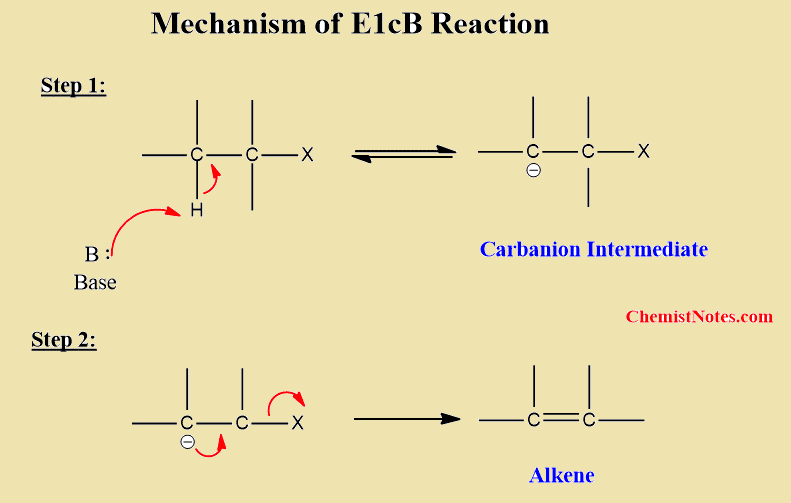
E1cB reaction mechanism
It is a two-step process. In the first step, hydrogen is removed from β-hydrogen to form a carbanion intermediate. In the second step, removal of leaving group takes place to form an alkene.
Although the rate-determining step in the E1cB mechanism is normally the unimolecular departure of leaving group from the carbanion intermediate, the overall reaction rate is second order.
Rate = k [RX] [Base]
E1cB reaction conditions
This reaction mechanism does not occur in all substrates, rather it occurs in that substrate has the following features.
- substate must have highly acidic hydrogen.
- the substrate must have poor leaving groups like OH–, CN– etc.






