Table of Contents
ToggleChemical equilibrium, its definition, fascinating characteristics, examples of some reactions in equilibria, and two main types of chemical equilibria with examples have been discussed here. Let’s explore.
Chemical equilibrium
Most chemical reaction involves the complete conversion of reactant to product and is spontaneous under suitable sets of conditions. But, there are many reactions that don’t undergo completion because the reactants are not completely converted to products. This is due to the fact that the products formed are also combined together and converted into reactants. The conversion of reactant to product and product to reactant occur simultaneously and a state is reached where the concentration of reactant and product becomes constant. This state is known as a chemical equilibrium.
Chemical equilibrium definition
Chemical equilibrium is defined as a state of equilibrium in which the rate of forward reaction and the rate of backward reaction becomes exactly equal. In another word, chemical equilibrium is a state in which measurable properties of the system don’t undergo any noticeable change under a particular set of conditions.
Chemical equilibrium is represented by the reversible arrow (⇌). For example:
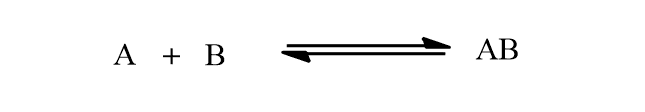
The only reversible reaction can exist in chemical equilibrium but an irreversible reaction can not exist because it undergoes completion in one direction.
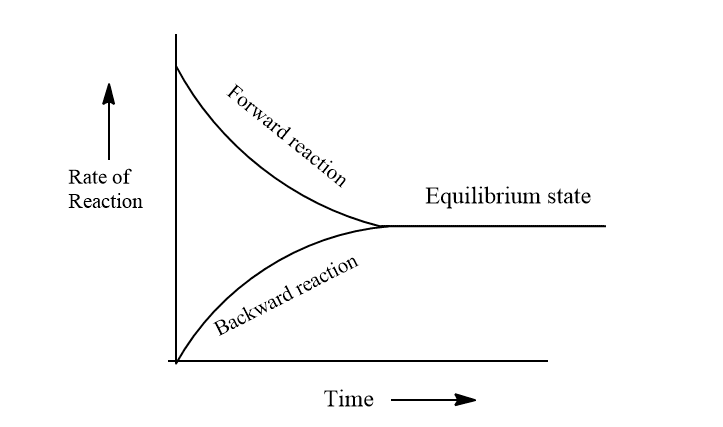
Characteristics of chemical equilibrium
Some characteristics/properties of chemical equilibrium are as follows:
- The observable properties of the system such as temperature, pressure, concentration, etc. become constant at equilibrium.
- The equilibrium can be attained only if the system is closed. If the system is open, the formed products can escape from the system, and equilibrium is not attained.
- There is no effect of catalyst on the equilibrium point. But, catalysts only enhance the rate of the forward and backward reaction to the same extent.
- The equilibrium can be approached from either direction.
Types of chemical equilibrium
There are two types of chemical equilibrium viz. homogeneous and heterogeneous.
Homogeneous equilibrium
The chemical equilibrium in which all the reactants and products are present in the same phase is called homogeneous equilibrium. For example:
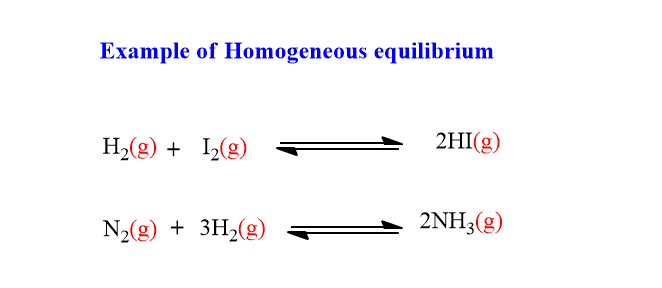
The g in the bracket indicates the gaseous state of reactants or products. In this example of the formation of hydrogen iodide, the product HI and reactants all are in the gaseous state. Hence, it is an example of homogeneous equilibria.
Heterogeneous equilibrium
Chemical equilibrium in which two or more phases are present at an equilibrium state is called heterogeneous equilibrium. For examples:
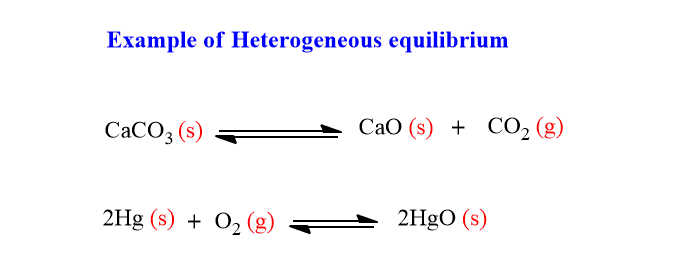
The letter s in the small bracket in the above chemical equation indicates the solid state of chemical species. Since reactants and products are in different states, it is termed heterogeneous equlibria.