Table of Contents
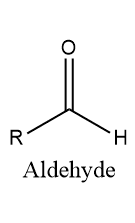
ToggleAn aldehyde is an organic molecule that contains a functional group with the formula RCH=O. These are neutral compound contain reactive carbonyl compound. In most reaction aldehydes are more reactive than ketones.
Test for Aldehydes
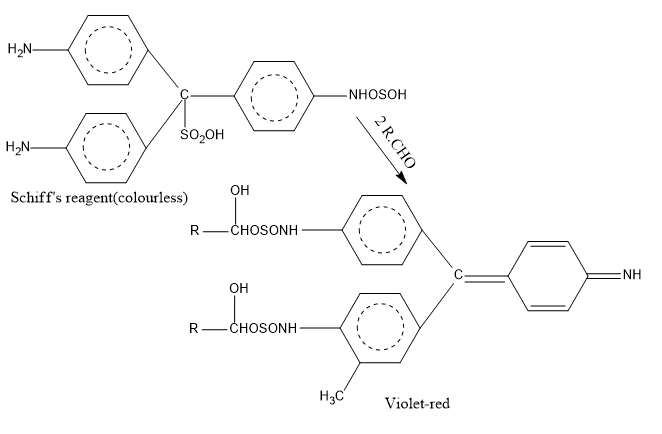
Schiff’s reagent test
Add 2 ml schiff’s reagent (Rosaniline hydrochloride decolorised with SO2) to 2 ml of liquid substance or 2 ml cold aqueous solution and mix for 2 minutes. Do not warm or heat. The presence of an aldehydic group is indicated by a deep violet-red or red color.
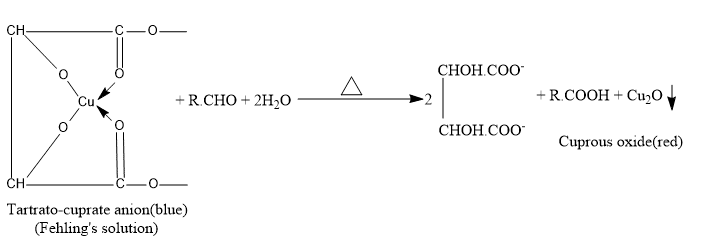
Fehling’s test
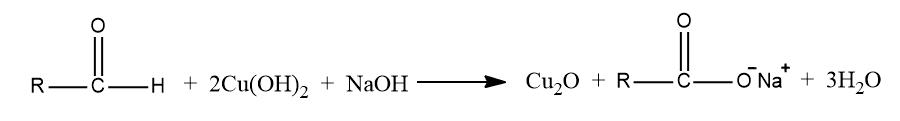
1 mL each of Fehling A (CuSO4 in water with a few drops of acetic acid) and Fehling B(Rochelle salt and NaOH solution) mix in a test tube. Boil for 5 minutes after adding 0.1 g or 2-3 drops of substance. Fehling’s blue color fades with time, leaving a reddish brown ppt of CU2O, obtaining the presence of an aldehydic group.
Benedict’s solution test
This is a modification of Fehilng’s solution test and consists of alkaline cupric ions complexed with cirrate ions.
In a test tube, put 4 mL Benedict’s reagent and a few drops of the unknown ( or a solution of the solid in ethanol or water). Bring the mixture to a boil. The formation of a yellow to red cuprous oxide precipitate indicates a positive test. he test is given only by aliphatic aldehydes but the reagent is not capable of oxidizing the aromatic aldehydes such as benzaldehyde. Thus it serves to distinguish between aliphatic and aromatic aldehydes.
Tollens’ test
In a test tube, add 2 mL Tollens’ reagent (Tollens’s reagent or ammonical silver nitrate is made by adding NaOH solution to AgNO3 until a white precipitate appears and this white precipitate that has been dissolved in excess NH4OH. Add 2-3 drops or 0.1g of substances to this white precipitate. Keep the test tube in boiling water for 5 minutes without stirring until a shining silver mirror forms on the test tube walls, indicating the presence of the aldehydic group.