Table of Contents
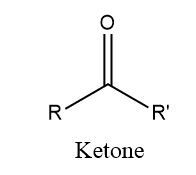
ToggleKetones are organic molecule that contains a functional group with the formula RCOR. These are neutral compound contain reactive carbonyl compound. In most reaction aldehydes are more reactive than ketones.
Test for Ketones
Many of the same reactions that are described in the classification of aldehydes can also be used to classify ketones.
Iodoform test
Disso0.1g of an unknown compound in 2 mL of water ( methanol or dioxane for water insoluble substance). Dropwise add 1 ml of 10% sodium hydroxide solution and iodine-potassium iodide reagent until a distinct dark color iodine persists. Allow it to stand at room temperature for a few minutes. If the color fades, apply additional reagent until the color reappears. Allow for a 15-minute stand time. A positive test results in the production of a yellow precipitate of iodoform (m.p 120oC) with a distinct odor. If the substance is insoluble in water then t the end of the reaction dilute with 10 ml water.
This reaction takes place through the formation of a carbanion, therefore the hydroxyl ions should always be present in excess.
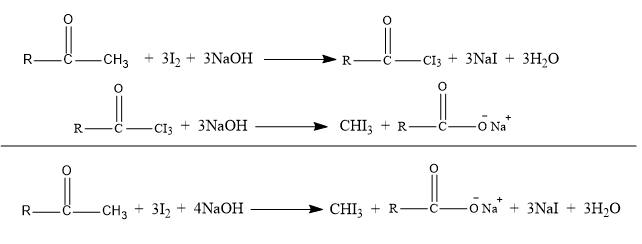
Aliphatic ketones. mixed ketones and alcohols also responds this test.
m-Dinitrobenzene test
The test is also preferred for methyl ketones. Add 1 % ethanolic m-dinitrobenzene solution and 2 drops of dil. sodium hydroxide solution to a dilute solution of the unknown ketone in ethanol. The formation of a red color is noticed. Acetone, methyl ethyl ketone, and acetophenone produce a red color. Benzophenone and benzaldehyde produce a faint red color at first, which darkens over time. Salicylaldehyde gives a yellow color in the beginning which turns pink on standing. Since m-dinitrobenzene itself gives a pink color with sodium hydroxide solution sample may be prepared for color comparison.
Others test for ketones
If the tests of aldehydes are negative perform following test for ketones.
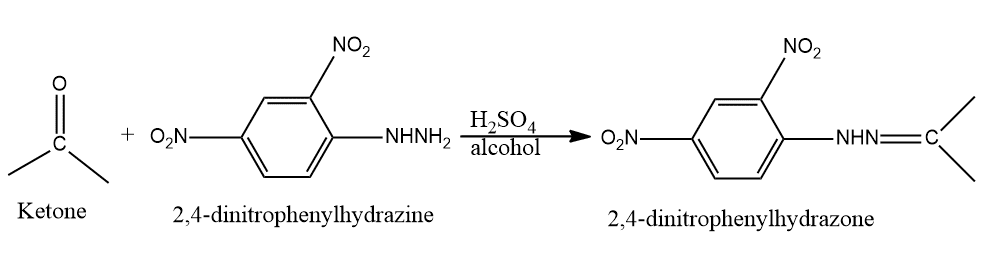
Add 2 ml of 2,4-dinitrophenylhydrazine solution in dilute HCl to a 0.5g or 0.5ml substance solution in 5 ml dilute HCl, cool, and allow to stand for 2 minutes when a yellow, orange, or red-colored crystalline precipitate (in case Schiff’s tests are negative) indicates the presence of a ketonic group.
Add 1 ml freshly prepared sodium nitroprusside solution and then drop by drop NaOH solution to 2-3 drops of substance. The presence of a ketonic group is confirmed by the presence of a wine red or orange red color.