Conformation denotes the particular structure of a molecule and n-pentane conformation is quite complex. Conformation relates to the different spatial arrangement of atoms or groups obtained by rotating a single bond between carbon and carbon. Different conformations of a given molecule differ in their potential energies due to the angle of torsion or dihedral angle.
Conformation of n-pentane
In n-pentane, several conformations are achieved by rotating either the C(2,3) or C(3,4) bond. This represents a rotation around two CH2-CH2 bonds. There are nine different conformations that can be obtained by rotating a single bond between this carbon and carbon; the trans conformation is marked by (a), and the gauche conformation by (g). The gauche conformation is further specified whether the torsional angle is near +60o or -60o which are designated as (g+) and (g–), respectively.
The nine staggered conformations of n-pentane are given below:
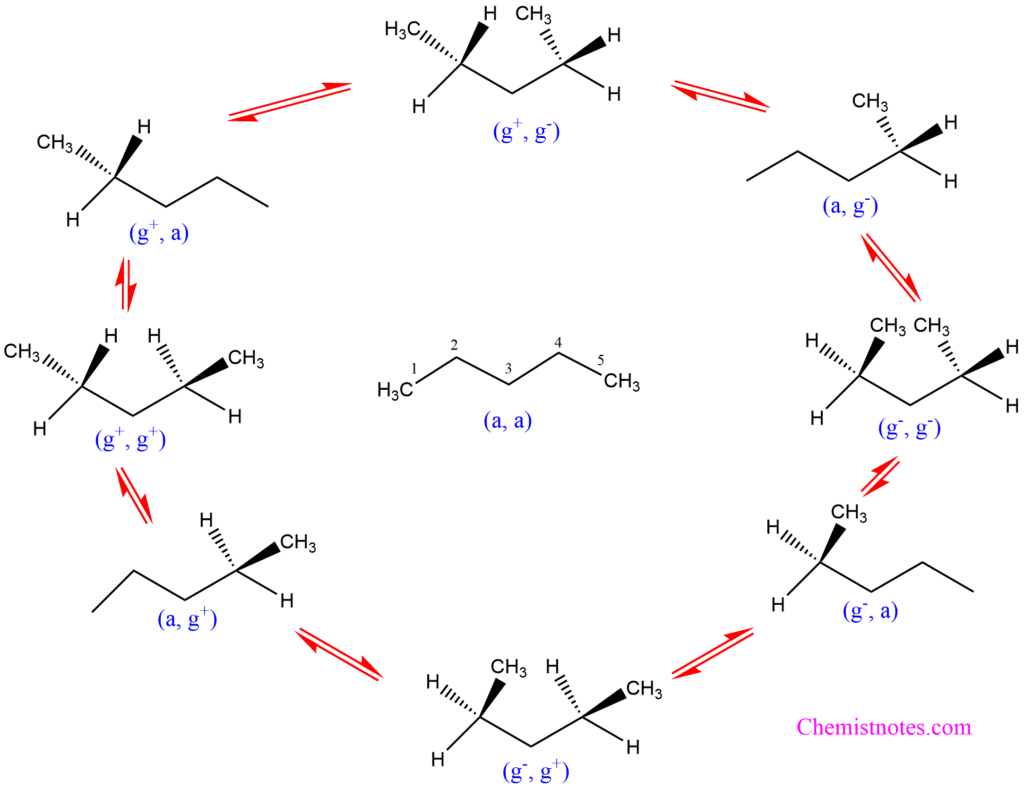
In the conformation (a, a) in the above figure, the single bond between carbon number 2(C2) and carbon number 3 (C3) when rotated 60o in an anticlockwise direction, the conformation (g–, a) is obtained. Similarly, by rotating a single bond between C3 and C4 to 60o in a clockwise direction or near +60o, the conformation (a, g+) is obtained. In the same way. by the rotation of the carbon-carbon single bond between C2 and C3 or C3 and C4 or both, the above conformations are obtained.
The conformations above and below (a, a) in the above figure are identical. The molecule (g+, g–) when rotated 180o becomes identical with (g–, g+). The compounds (a, g–) and (g–, a) are identical. One of these compounds can be rotated 180o to have the identical compound of the other. The same is the case with conformers (g+, a) and (a, g+). However, the compounds remaining left and right of the vertical line are enantiomers. That is, (g+, a) and (a, g–) are enantiomers. Enantiomers are different compounds but contain equal energies. Again, (g+, g+) and (g–, g–) are enantiomers. They are also of equal energies. If we see the identical basis, there are only six distinct conformations:
- (a, a)
- (g+, g– and g–, g+)
- (a, g– and g–, a)
- (g+, a and a, g+)
- (g–, g–)
- (g+, g+)
On the basis of enthalpy, n-pentane possesses only 4 confirmations:
- (a, a)
- (g, g)±
- (ag± or g±a)
- (g–, g– or g+, g+)
The calculated conformation distribution at 298.16K in liquid pentane is aa (34.6%), 54.6% (a, g), 10.8% (g, g), and less than 0.4% for (gg)±
n-pentane conformation video






