Table of Contents
ToggleClassification of Amino acids on different bases such as structure, polarity, nutritional requirement, and metabolic rate has been done with suitable examples.
Amino acids are a group of organic compounds containing two functional groups viz.-NH2 group and -COOH group. Amino acids are monomers of proteins. These are building blocks of proteins.
Since the amino acids contain an amino group and carboxylic acid groups, it enables amino acids to link together. Alpha-amino acids special type of amino acids in which these two functional groups are separated by exactly one carbon or both functional group is attached to the same carbon atoms.
General formula of amino acid
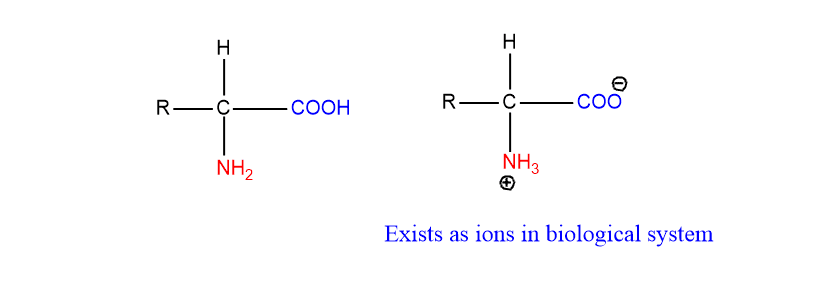
If both functional groups are attached to the same carbon atom, these are termed alpha-amino acids. Amino acids mostly exist in the ionized form in the biological system as shown below.
Optically active amino acid
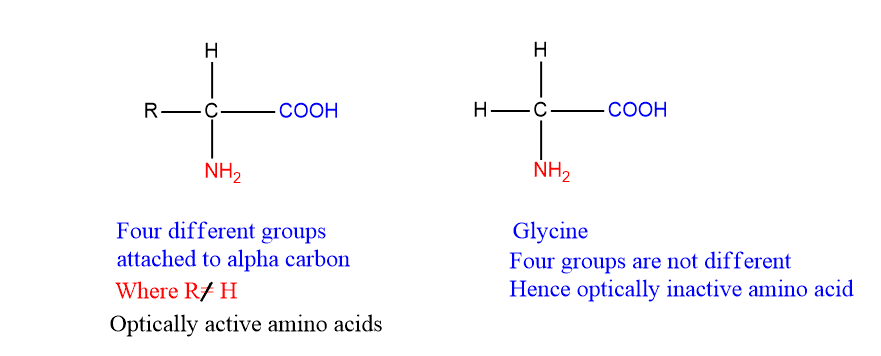
If a carbon atom is attached to four different groups, it is asymmetric and therefore exhibits optical isomerism. All the amino acids except glycine possess four different groups viz. R, H, COO– and NH3+ attached to the alpha carbon. Therefore, all the amino acids except glycine have optical isomers
Glycine does not have optical isomers because it does not possess four different groups as shown below: Hence, the optically inactive amino acid is glycine.
Amino acids can be classified in different ways based on structure, chemical nature, nutritional requirement, metabolic rate, etc.
Classification of amino acids based on structure
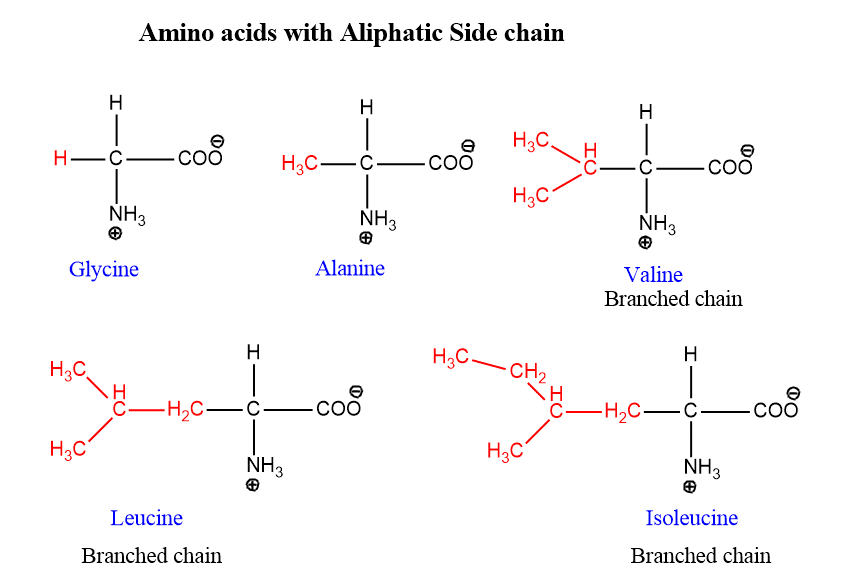
Amino acids with aliphatic side chain
These are the most simple amino acids having one amino and one carboxylic acid. For example, Glycine, alanine, valine, leucine, and isoleucine are the simplest amino acids.
Leucine, Alanine, and Valine contain branched aliphatic side chains. Hence also called branched-chain amino acids. The simplest amino acid is glycine.
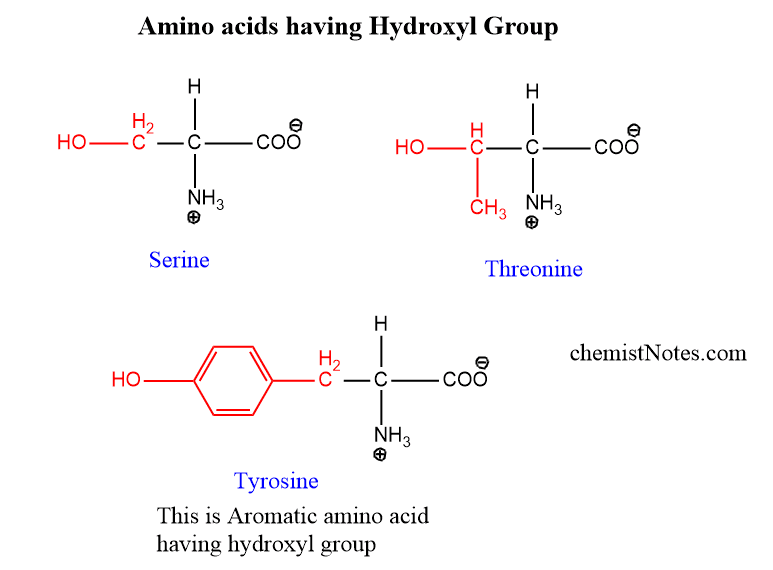
Hydroxyl group-containing amino acids
There are some amino acids in which the hydroxyl group is also present. Serine, threonine, and Trysosine are hydroxyl group-containing amino acids. But, Tyrosine is aromatic in nature therefore, it is considered an aromatic amino acid.
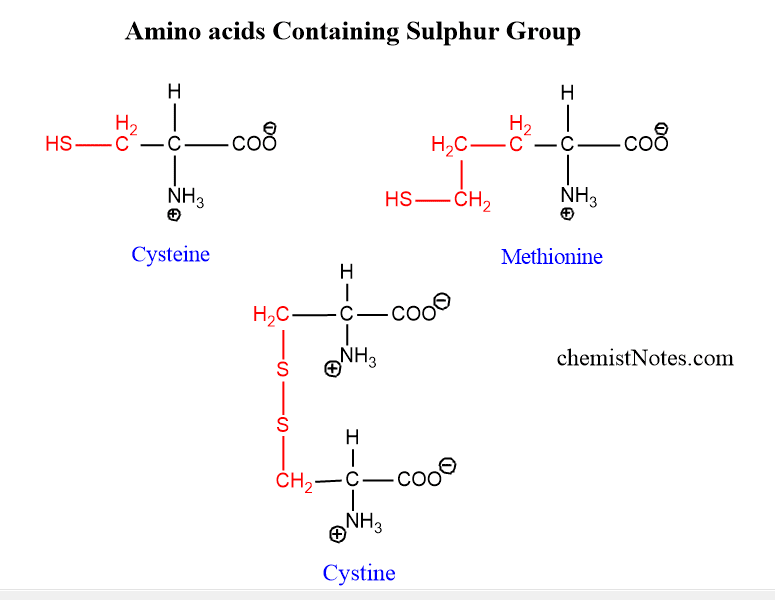
Sulphur containing amino acid
Cysteine, Methionine, and cysteine are sulphur-containing amino acids. Cysteine contains a sulfhydryl group and methionine contains a thioether group. Cystine is formed by the condensation of two molecules of cysteine.
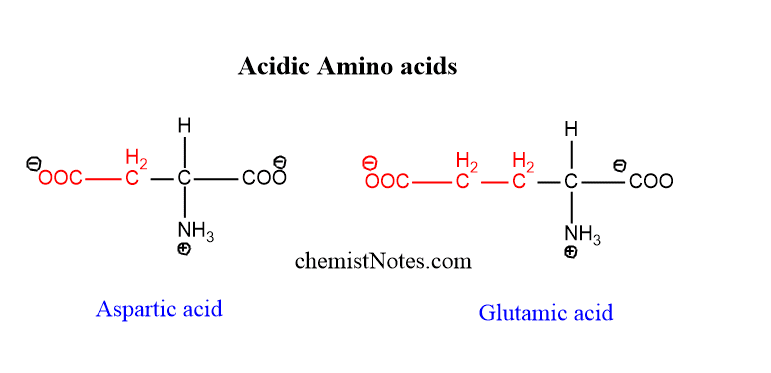
Acidic amino acids
Aspartic acid and glutamic acids are acidic amino acids in which two carboxylic acids and one amino acids are present. Asparagine and glutamine are their respective amide derivatives.
example of acidic amino acid
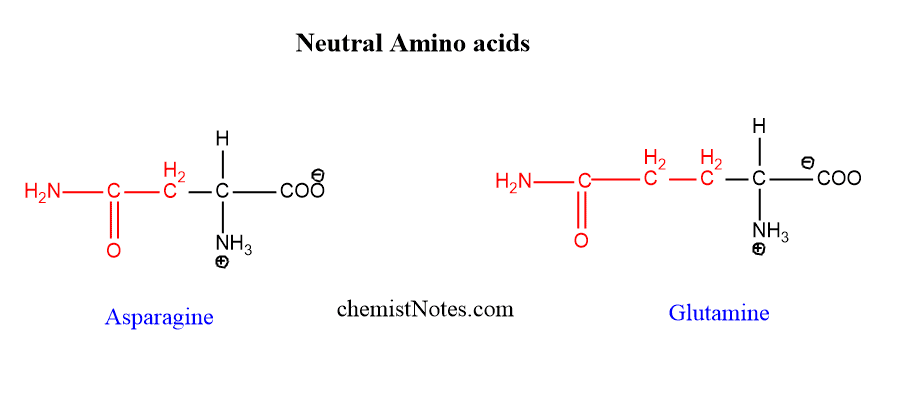
Neutral amino acids
Those amino acids which don’t have a charge are called neutral amino acids. If the number of the amino groups and carboxylic acid groups is the same, then the amino acid is called neutral amino acids. Asparagine and glutamine are neutral amino acids.
example of neutral amino acid
Glutamine and Asparagine are two neutral amino acids
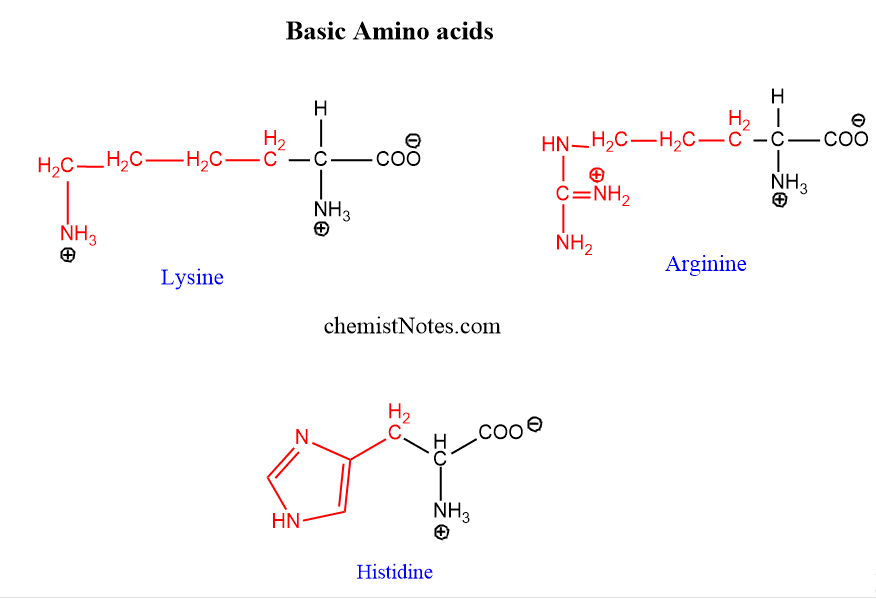
Basic amino acids
Three amino acids lysine, arginine, and histidines are dibasic monocarboxylic acids. Arginine contains a guanidine group and histidine contains an imidazole ring.
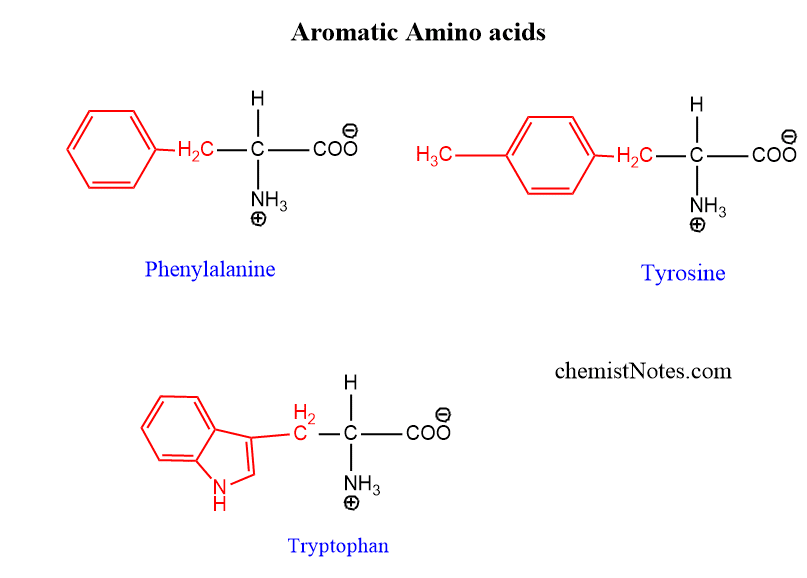
Aromatic amino acids
Phenylalanine, Tyrosine, and Tryptophan are aromatic amino acids. Tryptophan contains an indole ring.
aromatic amino acids examples
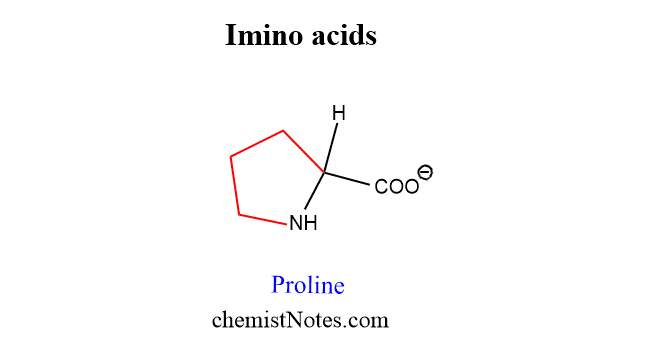
Imino acids
There is a unique amino acid that contains an imine group(=NH) instead of an amino group (-NH2). Therefore, these are also called alpha-imino acid. Example: Proline
classification of amino acids based on polarity
Amino acids are classified into four groups based on their polarity.
Non polar amino acids
These amino acids have no charge on the R group. These are also called hydrophobic, which means water-hating. Examples of non-polar amino acids are Alanine, Leucine, Isoleucine, Valine, Methionine, Phenylalanine, Tryptophan, and proline.
Polar amino acids with no charge one R group
These amino acids don’t have a charge on the R group but they contain groups such as hydroxyl, sulfhydryl, and amide and these make amino acids polar. These groups participate in hydrogen bonding of protein structure. Example: Simple amino acid glycine, Serine, Threonine, Cysteine, Glutamine, Asparagine, and Tyrosine.
Polar amino acids with positive R groups
Three amino acids lysine, arginine and histidine are included in this group.
Polar amino acids with negative R group
Dicarboxylic monoamine acids such as aspartic acid and glutamic acid are considered in this group.
Classifications of Amino acids on the basis of Nutritional requirement
Essential amino acids
The amino acids which can not be synthesized by the body and must be included in the diet are called essential amino acids. These are also called indispensable amino acids. There are 10 Amino acids essential for humans.
Examples of Essential amino acids are Arginine, Valine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, and Tryptophan.
Essential amino acids mnemonic
The mnemonic for essential amino acids is @M.PHIL AT VLT.

Semi essential amino acids
Some amino acids can be synthesized by adults and not by growing children, such are called semi-essential amino acids. Arginine and Histidine are two semi-essential amino acids.
Non-essential amino acids
Those amino acids which can be synthesized by the body and need not be consumed in the diet are called non-essential amino acids. These are also known as dispensable amino acids. The human body can synthesize about 10 amino acids. Examples: Glycine, Alanine, Serine, Cysteine, Aspartate, Asparagine, Glutamate, Glutamine, Tyrosine, and Proline.
Classification Of amino acids based on their Metabolism
The carbon chain of amino acids acts as a Precursor for the synthesis of glucose or fat or both. On the basis of such metabolism, amino acids are divided into 3 types.
Glycogenic amino acid
The amino acids which act as the precursor for the synthesis of glucose or glycogen are called Glycogenic amino acids. Examples: Alanine, Aspartate, Glycine. Methionine etc.
Ketogenic amino acids
The amino acids from which fat can be synthesized are called ketogenic amino acids. Example: Leucine and Lysine.
Glycogenic and Ketogenic amino acids
Some amino acids that can be used for the synthesis of glucose, as well as fat, are called glycogenic and ketogenic amino acids.
Examples: Isoleucine, Phenylalanine, Tryptophan, Tyrosine
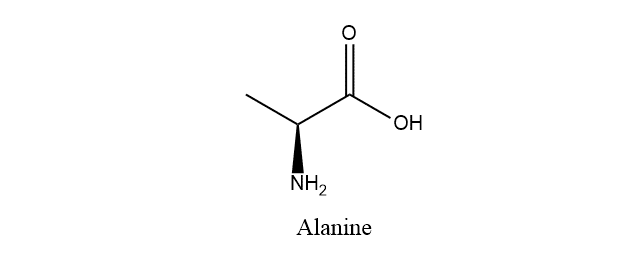
Draw the structure of amino acid alanine
List two sulphur-containing amino acid
Sulphur-containing amino acids are cysteine and Methionine. The structures of these amino acids are shown above.
References
- Lehninger, D. L. Nelson, and M. Cox Michael, Lehninger Principle of Biochemistry, (4th Edition), Macmillan Worth publisher, 2005.
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryrer, Biochemistry, (5th Edition),W.H. Freeman and Company, 2002.