Table of Contents
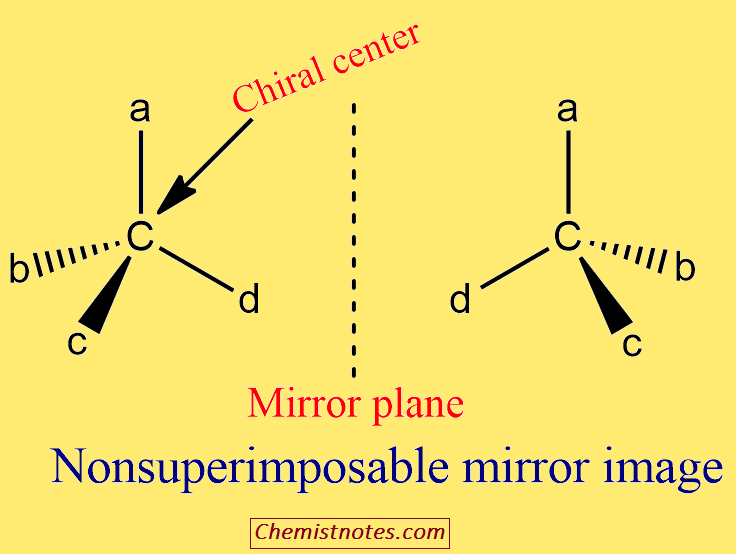
ToggleA chiral center is an atom with four different groups bonded to it in such a way that it has a non-superimposable mirror image. The molecules that are non-superimposable in their mirror image are called chiral molecules, and this property is known as chirality. If the molecule superimposable on its mirror image is an achiral molecule. Chiral carbons are also called as asymmetrical carbons.
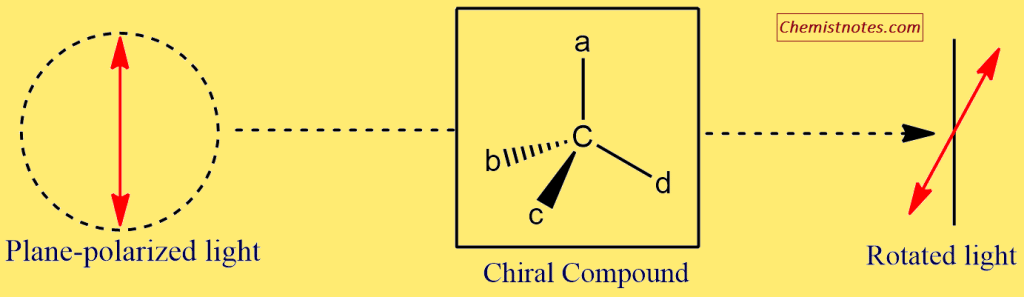
Chiral molecules are optically active, which rotate the plane of polarised light. Despite the fact that pure compounds are always optically active if they are composed of chiral molecules.
How to determine the Chiral Center?
- Chiral centers are carbon atoms that are attached to four different groups or substituents and are placed at the corner of the tetrahedron.
- First, consider compound A, which has four different substituents attached to the carbon atom. Compound B is the mirror image of compound A.
- So, here, the next step is to identify the type of mirror image: is the compound superimposable or non-superimposable?
- To do this, rotate compound A around one axis of rotation and examine any of the rotation products that lead to compound B. If not, compound A is chiral.
- Let’s rotate compound A in the clockwise direction.
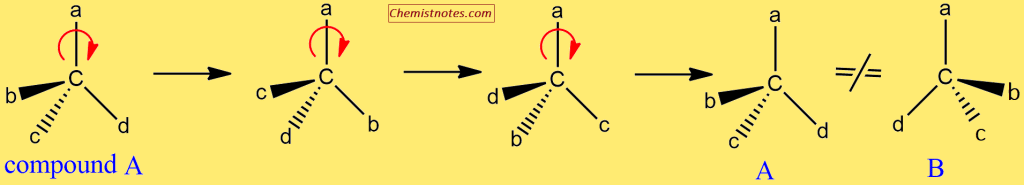
As you can see, none of the structures above are the same as the mirror image, compound B. As a result, we can conclude that compound A is chiral.
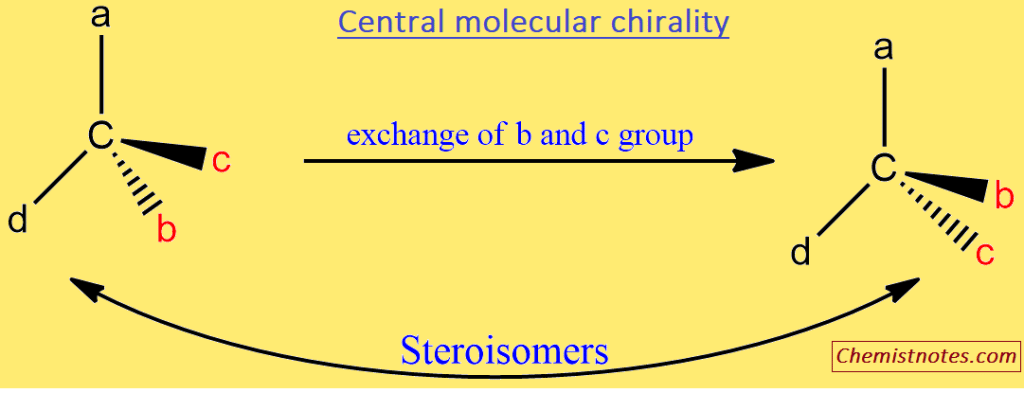
Central Molecular chirality
Stereoisomers are isomers that have the same bond connectivity but different configurations. In pairs, some of these bear a non-superimposable mirror image relationship with pairs. These are enantiomers. Since the enantiomers are members of the same point group, they can only be distinguished using chiral reagents or chiral agents, such as right- or left-circularly polarized light. For example, the two enantiomers rotate the polarized light’s plane in opposite directions but to the same extent. One rotates the light to the right (dextro), while the other rotates it to the left (levo). This phenomenon is known as “optical activity” due to central molecular chirality.
Axial Molecular Chirality
Allenes of the type XCH=C=C=CHX can reside as a pair of non-superimposable mirror image isomers, that is, enantiomers. This dissymmetry appears because of the near orthogonality of the two planes containing two C=C (comparable to the extended tetrahedron).
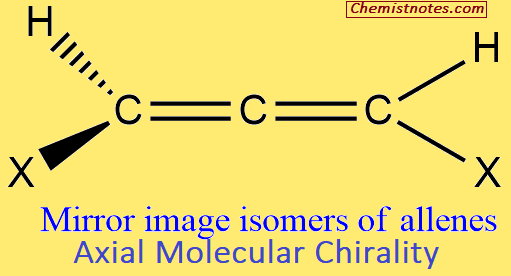
The axis C=C=C of molecules of the type abC=C=Cab is, therefore, a stereogenic axis. This phenomenon of optical isomerism is termed axial chirality.
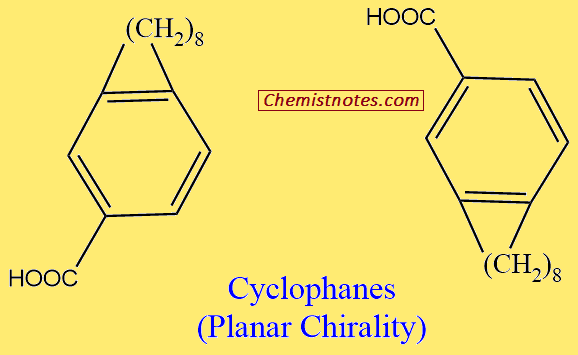
Planar Chirality
A dissymmetrically substituted planar molecule may be considered a two-dimensional chiral (2D-chiral). When the 1,4-positions of such 2D-chiral molecules are joined by a chain of carbon atoms to form a ring that can just slip through the plane, for example, cyclophanes, the swinging of the carbon chain across the planar portion of the molecule, can give rise to non-superimposable mirror image configurations. Such a chirality is termed planar chirality.
Helical Chirality
A helix possesses a C2-axis only, and it is essentially chiral, The right-handed and left-handed helices of a molecule represent two nonsuperimposable mirror images. Such chirality owes its origin to helicity. For example; phenanthrene is helically chiral.
FAQs
What is Chiral Center?
A chiral center is an atom with four different groups bonded to it in such a way that it has a non-superimposable mirror image.
How to find Chiral Centers?
Rotate compound A around one axis of rotation and examine any of the rotation products that lead to compound B. If not, compound A is chiral.
What does chirality mean?
The molecules that are non-superimposable in their mirror image are called chiral molecules, and this property is known as chirality.
Are meso compounds Chiral?
Meso compounds are achiral compounds with chiral centers.






