Table of Contents
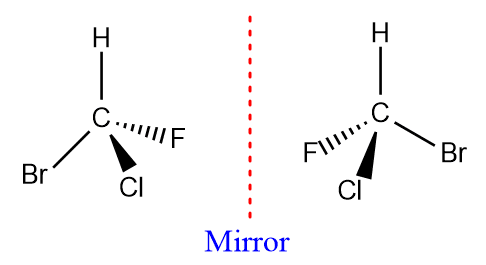
ToggleOptically active compounds are those that rotate the plane of polarized light. A pure compound that is optically active is not superimposable on its mirror image. A molecule that contains only one stereogenic carbon atom (a carbon atom linked to four different groups, also known as a chiral atom or an asymmetric carbon atom) is always chiral and thus optically active. In any situation of optical activity of a pure compound, there are only two isomers known as enantiomers, which differ in structure only in the left- and right-handedness of their orientation.
Plane polarized light
Light has certain properties that are understood by observing it as a wave phenomenon that occurs at right angles to the direction in which the light travels. There are an infinite number of planes that pass through the propagation line, and ordinary light is vibrating in all these planes. If we consider that we are looking directly into the beam of a flashlight, show schematically the sort of vibrations that are taking place, all perpendicular to a line between our eye and the paper (flashlight). The light that only vibrates in one of these possible planes is said to be plane-polarized light.
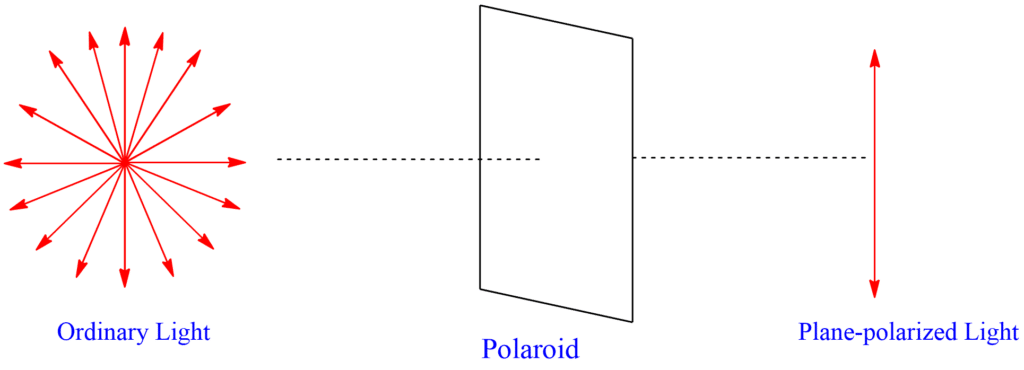
Ordinary light can be transformed into plane-polarized light by passing through a lens made of polaroid materials. When polarized light vibrates in one plane and is passed through an optically active material, it emerges vibrating in another plane.
Optically active vs inactive compound
The disymmetric molecule can rotate the plane of polarized light i.e. they possessss optical activity. It should be known that when light passes through a transparent material it interacts with the molecule of that material. The light is slowed by this interaction, this is mainly due to refraction of light.
For most compounds, because of the random distribution of the large number of molecules that make up even the smallest sample of a single pure compounds, for every molecule that the light encounters, there is another molecule oriented as the mirror image of the first, which exactly cancels its effect. The net result is no rotation, that is , optical inactivity.
In special case, a molecule whose mirror image is not just another, identical molecule, but rather a molecule of a different, isomeric compound. In a pure sample of a single enantiomer, no molecule can serve as the mirror image of another, there is no exact canceling-out of rotations, and the net result is optical activity. Thus the same non-superimposibility of mirror images that gives rise to enantiomers also is responsible for the optical activity.
Measurement of optical activity
A polarimeter is used to measure optically active compounds. It depends on various factors, like the length of the sample vessel, temperature, solvent, concentration for solutions, pressure for the gases, and the wavelength of light. Of course, rotations determined for the same compound under the same conditions are identical. The number of molecules in the path of the beam is proportional to the length of the vessel and the concentration or pressure. To make it possible for one value of α for a pure compound to be compared with another α for that compound taken under different circumstances.
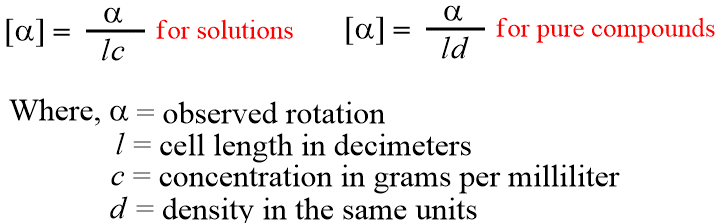
The specific rotation is usually given along with the temperature and wavelength of light used for the measurement, in this manner: [α ]T λ.
It must be emphasized that the value of α changes with conditions, but the molecular structure is unchanged. This finding is true even when the changes in condition are sufficient to change not only the amount of rotation but even the direction.
FAQs
What types of molecules display optical activity?
The molecule with chirality that possesses non-superimposability is the main type of molecule that show optical activity.
What does optically active mean?
Optically active compounds are those that rotate the plane of polarized light.
Are achiral molecules optically active?
Achiral molecules are optical inactive.
Are meso compounds optically active?
Meso compounds are optically inactive.
Are enantiomers optically active?
Enantiomers are optically active compounds.
Are diastereomers optically active?
Diastereomers are optically inactive.






