Table of Contents
ToggleA dye is generally a colored organic compound used to color a substrate such as paper, cloth, leather, or plastic. The human population has always been attracted by color for both aesthetic and ethical reasons. A dye must have an attractive color and attach itself to the substrate. The color is not readily altered by washing, heat, light, or other factors to which the material is likely to be exposed. Most dyes are organic compounds i.e they contain carbon.
There are some substances that are themselves not colored but they brighten the color imparted by another dye, or they whiten a white substrate. These optical brighteners or whiteners are termed white dyes.
The Chemistry of Colored
If a substance reflects all the wavelengths in the frequency range of 750–400 nm of electromagnetic radiation, then it appears white to our eyes. And if it absorbs all the wavelengths, it is a black substance. But if it absorbs all the wavelengths except the one that it reflects, then the substance will attain the color of the reflected light. On the other hand, if the substance absorbs only one wavelength and reflects all others, then it will attain the complementary color of the absorbed wavelength. For example, if it absorbs only blue light, it will appear yellow. In short, a substance will appear colored only when it absorbs some wavelengths in the visible range of the spectrum.
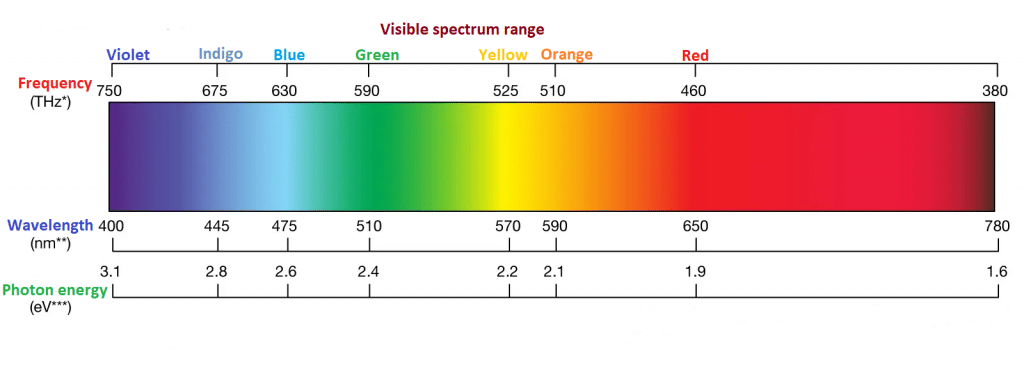
When it is said that a substance absorbs visible wavelength, it means that the molecules of the substance absorb photons of the visible light one photon per molecule.
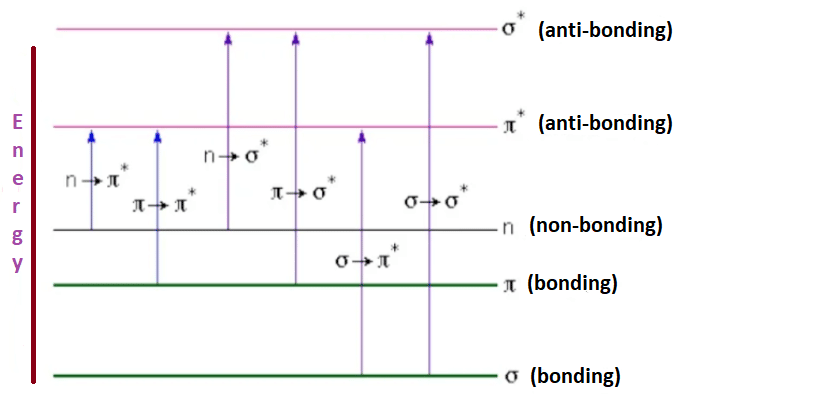
Dyes contain chromophore and auxochorome groups. Dyes give color due to the presence of chromophores. Some important chromophores are NO2, NO, N-N, C=O, and C=S. An organic molecule containing such functional groups absorbs photons of visible light to undergo π — π* or n — π* electronic transition. These compounds are colored compounds.
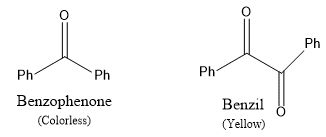
For example, Nitrobenzene is pale yellow, azobenzene is yellow-orange, and nitosobenzene is green color. Such multiple-bonded groups that are capable of imparting color too organic molecules are called independent chromophores and the colored compounds are known as chromogens. There are a few more multiple-bonded functional groups like =C=O and =C=C=, which alone cannot impart color, but in combination with either itself or with other chromophores can impart color to the molecules. These are called dependent chromophores. For example, Benzophenone is colorless, but benzil is yellow in color.
Conjugation of unsaturated groups in organic molecules plays an important role in producing color or deepening of color. For example, ethylene is colorless but when five C=C groups are in conjugation, the resulting compound is colored.
Classification of dyes
Very common classification of the dyestuff is based on the source from which it is made. Accordingly, the classification could be:
- Natural Dyes
- Synthetic Dyes
Natural Dyes
Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources. E.g. roots, berries, bark, leaves, and wood, and the other organic sources include fungi and lichens.

Synthetic Dye
Almost all the colors that you see today are synthetic dyes. Synthetic dyes are used everywhere in everything from clothes to paper, from food to wood. This is because they are cheaper to produce, brighter, more color-fast, and easy to apply to fabric.
Examples: Acid dyes, Azo dyes, Basic dyes, Mordant dyes, etc.

Azo Dyes
Among the synthetic dyes, azo dyes constitute the largest group containing nitrogen as the azo group -N=N- as a primary chromophore. These dyes are widely used in the food, pharmaceutical, cosmetic, textile, and leather industries. These dyes are highly colored and are prepared by diazotizing an aromatic amine and coupling it with a suitable aromatic compound. The chromophoric group in this class of dyes is one or more aromatic azo groups.
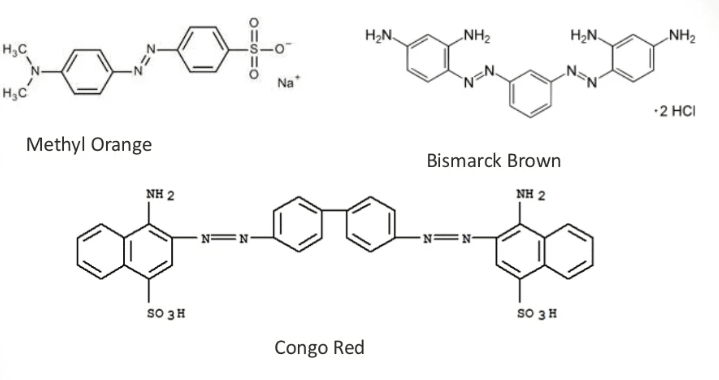
About 60–70% of the colors used in food and textile manufacturing are azo dyes. Azo dyes can produce a broad spectrum of colors, but yellow/red dyes are more common than blue/brown dyes.
Application of Dyes
- Dyes are applied to cellulosic fibers and blends of cellulosic with polyester, nylon and acrylics.
- Some indigo dyes are used for dyeing wool and silk.
- A wide range of organic leuco dyes are available, changing color between -5 oC and 60 oC.
- Novelties such as bath toys, color-changing spoons, mugs, and cups.

5. Microwave temperature indicators on food packaging.
6. Hair color

7. Thermal paper







