Table of Contents
ToggleElimination happens when the nucleophile attacks hydrogen instead of carbon. Hughes and Ingold proposed the E1 elimination Reaction for the reaction that proceeds by first-order kinetics. In this mechanism, the electronic changes, the bond-breaking, and the bond-making are the same as in E2; however, they occur one after the other, rather than simultaneously.
Mechanism of E1 reaction
E1 (unimolecular elimination reaction) involves two steps:
- In step 1: the substrate undergoes slow heterolysis to form halide ion and a carbocation.
- In step 2: the carbocation rapidly loses a proton to the base and forms the alkene.

The first step is the same as the first step in SN1. The carbocation reacts with a nucleophile in step 2 of SN1 to produce the substitution product; in step 2 of E1, the carbocation reacts with the base to produce the elimination product. The E1 reaction, like the SN1 reaction, exhibits first-order kinetics for the same reason. Only the slow first step determines the overall rate of reaction. Except for the many necessary solvent molecules, this rate-determining step involves only the substrate, and its rate depends only on the concentration of the substrate.
The rate of an E1 reaction is independent of base concentration
rate = k[RX]
E1 mechanism example
E1 elimination of t-BuOH in H2SO4
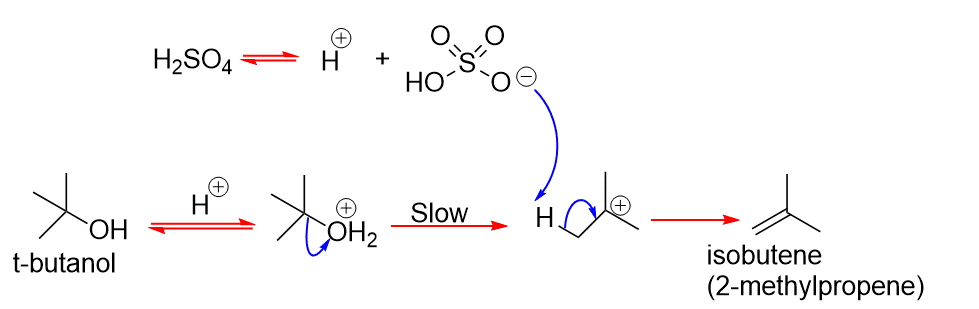
The HSO4 anion does not participate in the rate-determining formation of the carbocation and is also a poor nucleophile, so it does not attack C atom of the carbocation.
Evidence for the E1 Mechanism
- E1 reaction follows first-order kinectics
- E1 reaction are not accompained by a primary hydrogen istopes
- Show the same effect of structure on reactivity as SN1 reactions do; and
- where the structure permits, are accompained by rearrangement.
Reactivity in E1 reaction mechanism
In E1, reactivity is determined by the rate of formation of the carbocation and this depend upon the stability of the carbocation. Where the structure permits, these first-order eliminations are accompained by rearrangement. The double bond appears in places remote from the carbon that held the leaving group.
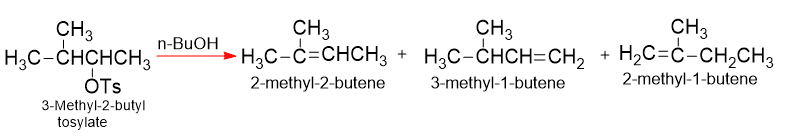
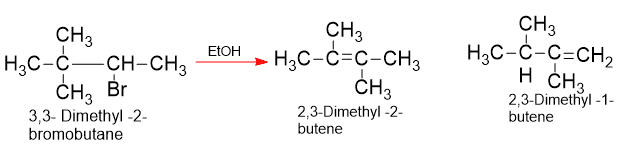
Refrences:
- March, J. Advanced Organic Chemistry, Reactions, 5th Edition; McGraw-Hill: New work, 1985.
- Indinavir synthesis: I. W. Davies and P. J. Reider, Chemistry and Industry (London), 1996, 412–15.
- G. Casiraghi, G. Casnati, G. Puglia, and G. Terenghi, J. Chem. Soc., Perkin Trans. 1,1980, 1862–65.






