Table of Contents
ToggleHow to read UV spectrum? In this blog, we have tried to provide some useful tricks related to UV spectra interpretation. UV spectra alone can not provide lots of information, i.e. it is difficult to extract lots of information from UV spectra alone. The UV spectra are very useful only when we already have a general idea of the structure. That means UV spectra do not give complete information about the structure of unknown compounds.
However, several generalizations can be made to assist in analyzing UV data. These generalizations, when combined with the IR and NMR data, become more fruitful.
How to read UV spectrum?
The generalizations or tricks to analyze the UV data are given more systematically.
Step 1: Look at a single band below wavelength 220 nm
If there is the presence of a single band with low to medium intensity (ε= 100 to 10,000) at a wavelength less than 220 nm, then we can predict the occurrence of an n→σ* transition. Generally, amines, alcohols, ethers, and thiols show n→σ* transition in this region provided the nonbonded electrons do not participate in the conjugation.
Exception: The cyano group(-C≡N) involves n→π* transition in this region but the intensity is very low(ε<100). This group can be confirmed using IR spectra.
Step 2: Look at a single bond of low intensity in the range of 250 to 360 nm
If you observe a single band of low intensity (ε= 10 to 100) in the range of 250 nm to 360 nm, with no major absorption at shorter wavelengths (200 to 250 nm), then we can conclude the occurrence of an n→π* transition. Such bands indicate the presence of a simple, or unconjugated, chromophore such as C=O, C=N, N=N, -NO2, -COOR, -COOH, OR -CONH2. This can be further confirmed by using IR and NMR spectra.
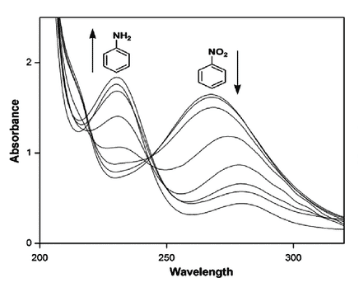
Step 3: Look at the two bands of medium intensity above 200 nm
If you observe two bands of medium intensity above 200 nm, that indicates the presence of an aromatic system. The presence of suitable substituents on the aromatic ring shows molar absorptivity above 10,000 if substituents increase the length of the conjugated system. Many polynuclear aromatic and heterocyclic compounds have very characteristic intensity and band shape (fine structure) patterns.
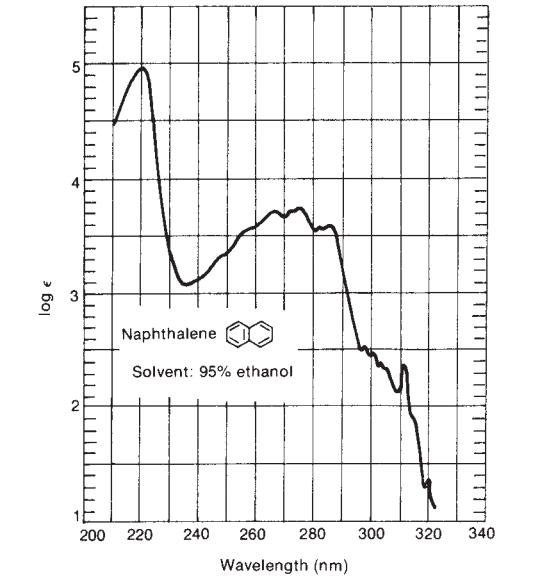
Step 4: Look at bands of very high intensity above 210 nm
If there is the presence of a band of very high intensity above 210 nm, we can predict the unknown compounds may be either α,β-unsaturated ketones, a diene, or polyene. The IR spectra of α,β-unsaturated ketones can further confirm. Similarly, the λmax value of diene can be calculated by using the Woodward-Fieser rule.
Step 5: Look at two bands below 300 nm
Simple ketones, acids, esters, and other compounds containing both π systems and unshared electron pairs show two absorptions of one n→π* transition at a longer wavelength below 300 nm, which is generally of two intensities, and another π→π* transition at a shorter wavelength below 250 nm, which is of high intensity.
Step 6: Look at the absorption band in the visible region
If compounds contain a long chain conjugated system or a polycyclic aromatic chromophore, these are likely to show absorption in the visible range( above 400 nm) and be colored. The nonaromatic systems, generally for four to five conjugated chromophores, also show absorption in the visible region.
In this way, the UV spectrum just gives little information about the chemical structure, but if we combine it with IR, Mass, and NMR spectral data, it becomes a great technique for structural elucidation.
You can also read: How to read IR spectra or NMR spectra?






