Table of Contents
ToggleDetermination of configuration of Cyclic compounds is almost the same as we already studied in our previous article which is the determination of the configuration of geometrical isomers by physical and chemical methods. They are almost the same as acyclic compounds but there is no free rotation in the case of cyclic compounds as they have fixed structures. They are hence based on symmetry condition which is called the symmetry-based method. Following are some methods to determine the configuration of cyclic compounds.
Determination of configuration of Cyclic compounds by Symmetry based method
They are explained in various methods below with suitable examples:
1. Testing resolvability
This is the first and most important chemical method to determine the configuration of cyclic compounds. As we know, cis gives Meso products and trans gives dl pairs and dl pairs are easily resolvable but Meso cannot be resolved. In acyclic compounds also when we take an example of tartaric acid, dl pair of tartaric acid can be resolved but Meso tartaric acid cannot be. Let us take an example of cyclopropane dicarboxylic acid, dl pair(enantiomer) of this compound can be resolved but Meso cant and can be determined as the cis and trans product.
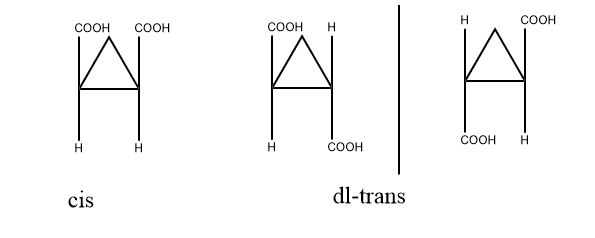
2. Chemical transformation
This is another method for the determination of the configuration of the cyclic compounds on the symmetry-based condition. For example decarboxylation of cis- 2,5-dimethyl cyclopentane-1,1-dicarboxylic acid gives two different Meso monocarboxylic acids whereas trans compound gives optically active or dl pair acid.
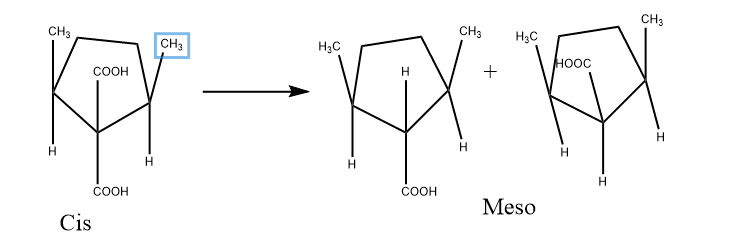
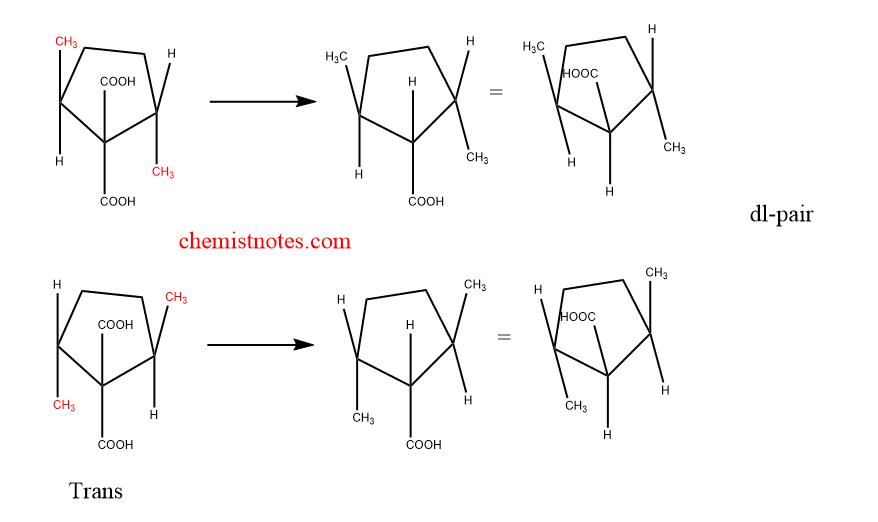
3. Ring Formation
As studied in acyclic compounds, cis can form acid anhydride easily but trans are not able to. Same as in the case of cyclic compounds, cis can easily form acid anhydride whereas trans cannot. Therefore, the ring formation method is useful to determine the cis and trans configuration of cyclic compounds.
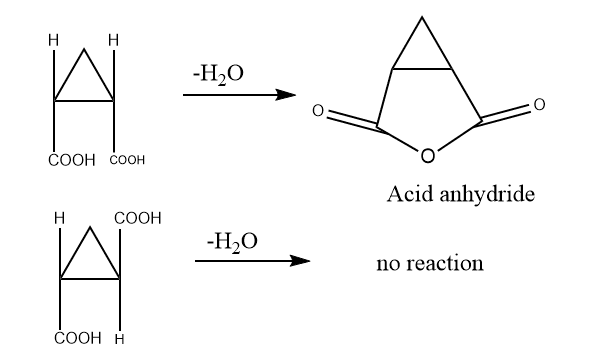
4. Mechanistic Consideration
This is an interesting method of symmetry-based method in which trans compound form epoxide whereas cis form ketone. In the cis position, for example, cis chlorohydrin changes very slowly into ketone. The reaction mechanism shows that if the product is epoxide then the compound is trans but when it is a ketone, it is cis.
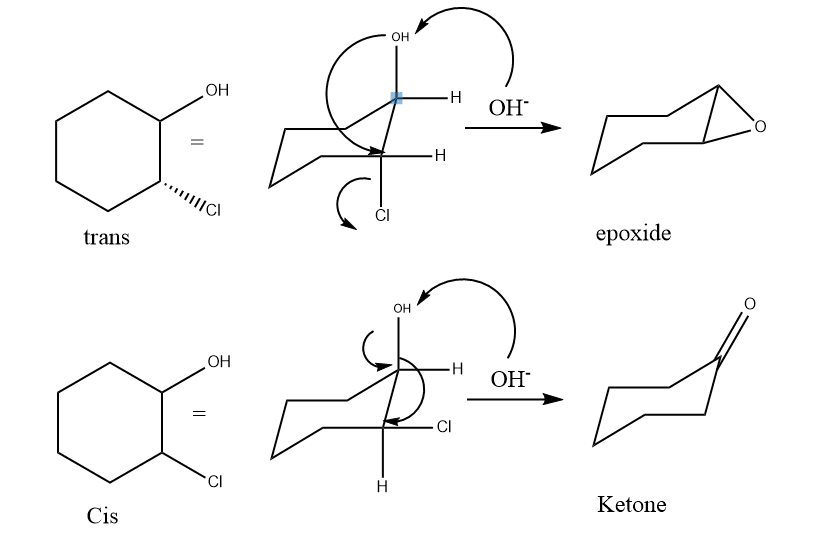
5. Physical methods
There is a number of physical methods same to acyclic compounds, for example, dipole moment, acid strength, different spectroscopic techniques, melting point, etc. Cis compound has a higher dipole moment than trans cyclic compounds as acyclic compounds. The acidic strength is higher for cis than trans as the PKA value for cis is lower than that of trans.
Stability of cyclic compounds
The stability of cyclic compounds depends upon angle strain, torsional strain or trannular effect, etc. The angle strain is higher for a small ring as the distortion angle is higher for such rings but in higher rings, the ring is stabilized by different conformations like boat form, chair form, etc
These are some chemical and physical methods to determine the configuration of the cyclic compound. You can also read our previously written article related to the determination of the configuration of cis and trans or acyclic compounds by chemical as well as physical methods. Keep learning.
FAQs/MCQs
What is configuration?
The spatial arrangement of groups or atoms at the carbon atoms is called configuration.
What is the different method for determining the configuration of the cyclic compound?
The different methods are test resolvability, chemical transformation, mechanistic consideration, ring formation, etc.
Which product can be easily resolvable by the symmetry method?
Trans for dl-pairs which are easily resolvable by the symmetry method.
Which isomers of the cyclic compound has higher acidic strength?
Cis isomers have a lower pka value and higher acid strength.






