Table of Contents
ToggleHeterolytic fission and Homolytic fission are among common ways of bond breakage. Most of the organic compounds are made up of covalent bonds in which pair of electrons are shared by two atoms. When the chemical reaction takes place, an event like breakage of the existing chemical bond, and the formation of a new bond takes place in most of the cases. This breaking of bond (covalent bond) is known as bond fission or lysis. Generally, bond fission takes place in two ways:
Homolytic Fission
Homo: equal; lytic/lysis: breaking [free radicals are formed]
Homolytic fission is a process in which the shared pair of electrons are retained by each of the fragmented atoms during the cleavage of a covalent bond. The process is also called homolysis or homolytic cleavage. When a neutral molecule undergoes homolytic fission, each of the bonded atoms retains one electron from the shared pair, and hence free radicals are produced.
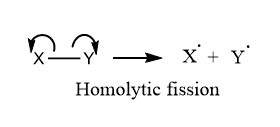
Conditions for Homolytic fission
Here are some of the conditions that favor the lysis of covalent bond by Homolytic fission:
- Bond between two atom must be non-polar i.e. there should be no differenc in electronegativity between the bonded atoms
- Presence of Heat, Electricity, Peroxide, and Radicals favors the formation of free radicals
Heterolytic Fission
Hetero: unequal; lytic/lysis: breaking [carbocations and carbanions are formed]
Heterolytic fission is a bond breakage process in which the shared pair of electrons are retained/taken by any one of the atoms and hence leads to the formation of positively charged species or carbocations, and negatively charged species or carbanions. This process is also called heterolysis or heterolytic cleavage. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms (that one which possesses greater electronegativity) retains a shared pair of electrons, and hence carbocations and carbanions are produced.
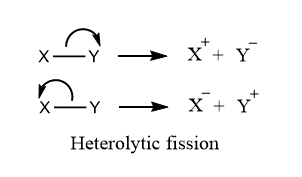
Conditions for Heterolytic Fission
Here are some of the conditions that favor the lysis of covalent bond by Heterolytic fission:
- Two bonded atoms must have a large difference of electronegativity i.e. the bond between two atoms must be polar
- Factors like low temperature, polar solvent, etc. favours the heterolytic cleavage
Differences between Homolytic and Heterolytic Fission
| Homolytic Fission | Heterolytic Fission |
| Homolytic fission is a process in which the shared pair of electrons are retained by each of the fragmented atoms during the cleavage of a covalent bond | Heterolytic fission is a bond process in which the shared pair of electrons are retained/taken by any one of the atoms |
| Free radicals are formed | Carbocation and Carbanions are formed |
| Bond between two atoms should have no difference in electronegativity | Two bonded atoms must have a large difference in electronegativity |
| Factors like Heat, Electricity, Peroxide, and Radicals favors the homolytic fission | Factors like low temperature, polar solvent, etc. favor the heterolytic cleavage |






