Table of Contents
ToggleHyperconjugation effect also called as Baker-Nathan effect is a permanent effect defined as delocalization of sigma (σ) electrons of C–H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p-orbital or π-orbital. It is due to the overlapping of σ-bonding orbital of the single bond or the orbital containing a lone pair with an adjacent π-orbital or p-orbital of the double bond. For the Hyperconjugation effect, there must be an α-CH group or a lone pair on the atom next to the sp2 hybrid carbon atom.
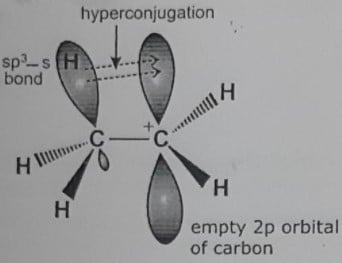
Hyperconjugation effect with examples
One of the most common examples of hyperconjugation effect is the stability of carbocation. Tertiary carbocation is the most stable in comparison to secondary, primary, and methyl carbocation. It is due to the Hyperconjugation effect in which delocalization of σ electrons of C-H bond of an alkyl group attached to an atom with an unshared p orbital takes place.
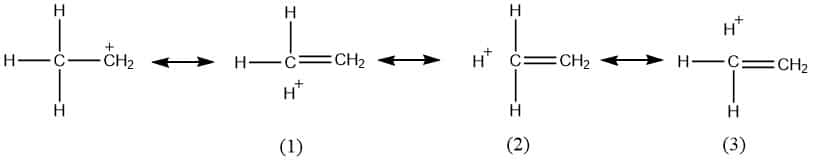
Here in 1o carbocation, structures (1), (2), and (3) are hyperconjugative structures that occur through α-CH group next to the double bond. As there is no bond between Hydrogen and carbon atom in these hyperconjugative structures, it is also called no-bond resonance.
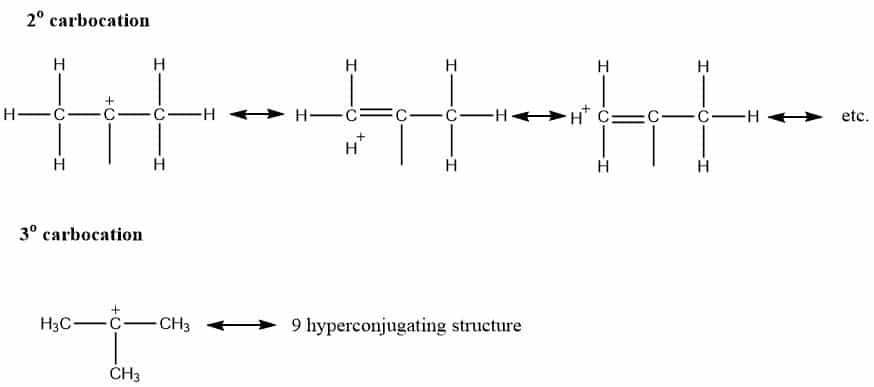
Similarly, there occur 6 hyperconjugative structures for 2o carbocation and 9 hyperconjugating structures for 3o carbocations as shown above. Greater the number of hyperconjugating structure, greater will be the stability. Thus, C-H bond of carbon group attached to carbon atom containing a positive charge stabilizes by donating electron density into a carbocation’s empty p-orbital. Therefore, the greater the alkyl group gets attached to the carbocation, the more will be the hyperconjugation effect, and hence greater will be the stability. Therefore, the stability order is Methyl carbocation < primary carbocation < secondary carbocation < tertiary carbocation.
Differences between Hyperconjugation effect and Inductive effect
| Hyperconjugation efffect | Inductive effect |
| Hyperconjugation effect is the delocalization of sigma (σ) electrons of C–H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p-orbital or π-orbital. | The polarization of one bond caused by the polarization of an adjacent bond is known as an inductive effect. |
| It involves both sigma bond orbitals and p-orbital or π-orbital. | It only involves only sigma electrons. |
| It is due to the overlapping of σ-bonding orbital or the orbital containing a lone pair with adjacent π-orbital or p-orbital of the double bond. | It is due to the polarization of bonds or the displacement of sigma electrons. |
| Leads to the stabilization of molecule via delocalization of π- electrons | It stabilizes the molecule by the transmission of charges through the molecules. |
Significance of hyperconjugation effect
Hyperconjugation effect several physical and chemical properties. Some of its effects are discussed below:
- Stability of alkene: Greater the number of alklyl group attached to the double bonded carbon, greater will be the stability of alkene. This is due to increase in the number of hyperconjugation structure or number of contributing no bond resonance structures.
- Stability of carbocation: The stability of carbocation via hyperconjugation effect is already discussed above.
- Stability of free radicals: This is due to the hyperconjugation effect, stability of free radical is in the order of methyl < primary < secondary < tertiary.
- Bond length: There occurs shortening of carbon-carbon single bond adjacent to the double bond due to the hyperconjugation efect.
- Dipole moment: Since the contributing structures show some extent of polarity, dipole moment of the molecules is greatly affected.
FAQs
Hyperconjugation effect in organic chemistry
Hyperconjugation effect is the delocalization of sigma (σ) electrons of C–H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p-orbital or π-orbital. One of the most common examples of hyperconjugation effect is the stability of carbocation






