Table of Contents
ToggleMolecular orbital theory was developed by F.Hund and R.S. Mulliken in 1932. According to molecular orbitals theory, all the atomic orbitals of combining atoms undergo mixing to form a new set of orbitals termed molecular orbitals.
Features of molecular orbital theory
The main features of molecular orbitals theory are:
- According to Molecular orbital theory, all the atomic orbitals of combining atoms first mix to form a new set of orbitals called molecular orbitals. Once the molecular orbitals are formed, the combing atoms lose their identity of atomic orbitals.
- The number of molecular orbitals formed is equal to number of atomic orbitals mixed.
- The molecular orbitals are formed by the linear combination of atomic orbitals.
- Molecular orbitals are formed by addition and subtraction overlap of atomic orbitals. The molecular orbitals formed by addition overlap are called bonding molecular orbitals and the molecular orbitals formed by substraction overlap are called anit-bonding molecular orbitals.
- Addition of electrons in bonding molecular orbitals tends to stabilize the molecule while addition of electrons in anti-bonding molecular orbital tends to destabilize the molecules.
- The shape of molecular orbitals formed depends on the shape of atomic orbital mixed.
- The atomic orbitals participating in the formation of molecular orbitals should have same symmetry and comparable energies.
- Molecular orbitals have different energy, shapes and sizes.
- Each electrons in the molecular orbitals can be described by its wave function Ψ and Ψ2 gives the probability of electrons density in the molecular orbitals.
- The bonding molecular orbital has lower energy and greater stability than anti-bonding molecular orbitals.
- The electrons in a molecule are present in various molecular orbital as the the electrons of atom are present in different various shells.
- In molecular orbitals, electrons are in influence of two or more nuclei.
- The molecular orbitals are filled in accordance to Aufbau principle, Pauli’s principle and Hund’s rule.
Molecular orbital theory bonding and antibonding
Bonding molecular orbitals
Bonding molecular orbital is formed by the positive overlap of atomic orbitals of combining atoms. The electron density is high between two nuclei. The formation of bonding molecular orbitals is shown below:
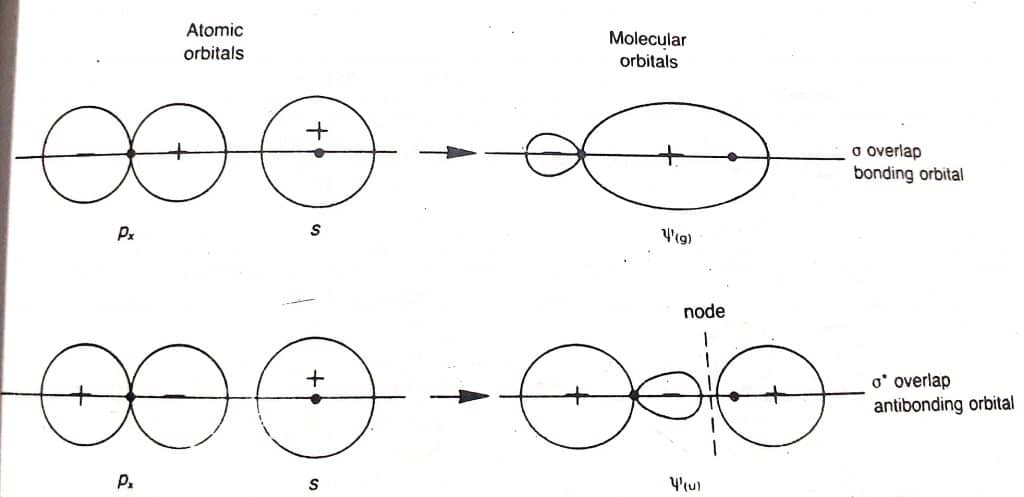
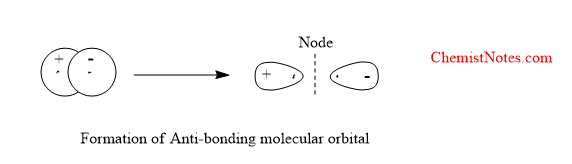
Anti bonding molecular orbitals
Anti-bonding molecular orbital is formed by the negative overlap of atomic orbitals of combining atoms. The electron density is very low between the two nuclei. The formation of antibonding molecular orbitals is shown below:
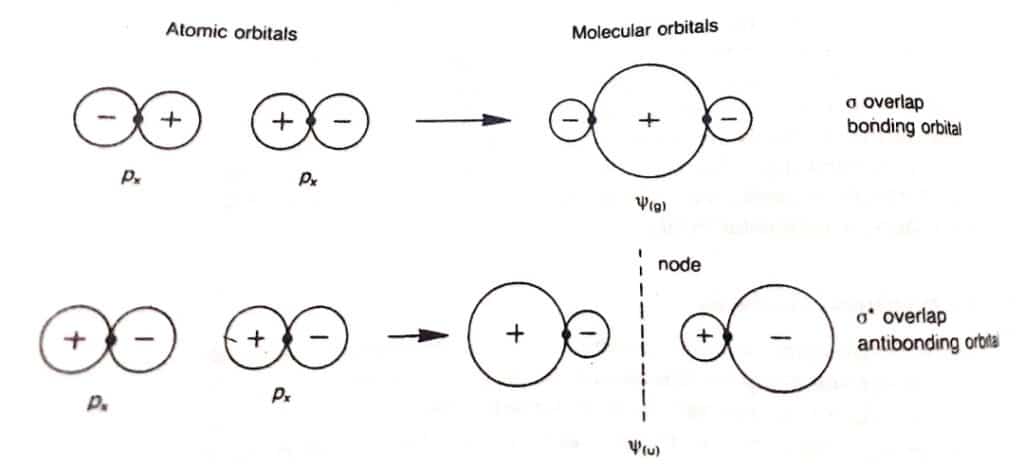
Non-bonding molecular orbitals
A molecular orbital in which the in-phase bonding atomic orbitals overlap equals the number of out-phase antibonding atomic orbitals. Electrons in a nonbonding molecular orbital are higher in energy than in a bonding molecular orbital, but lower in energy than electrons in an antibonding molecular orbital. The formation of non-bonding molecular orbitals is illustrated below;
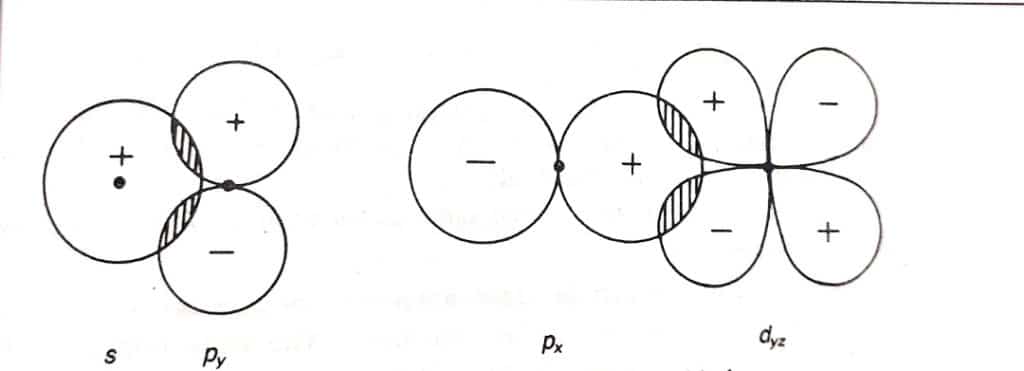
Difference between Bonding and anti-bonding molecular orbitals
The main difference between bonding and anti-bonding molecular orbitals are shown below:
| Bonding molecular orbitals | Anti-bonding molecular orbitals |
| It is formed by addition overlap of atomic orbitals and represented as Ψb=ΨA+ΨB | It is formed by subtraction overlap of atomic orbital and represented as Ψ*=ΨA-ΨB |
| It is formed by the overlap of atomic orbitals having the same sign. | It is formed by the overlap of atomic orbitals having the opposite sign. |
| In bonding molecular orbitals, the electron density between the two nuclei is high. | In anti-bonding molecular orbitals, the electron density between the two nuclei is very low. |
| It leads to the formation of molecules. | It leads to the breaking of molecules. |
| It has lower energy than atomic orbitals. | It has higher energy than atomic orbitals. |
| The addition of electrons in bonding molecular orbitals tends to increase the stability of molecules. | The addition of electrons in anti-bonding molecular orbitals tends to decrease the stability of molecules. |
LCAO method molecular orbital theory
According to LCAO method, molecular orbitals are formed by a linear combination of atomic orbitals which are brought by addition overlap and subtraction overlap. When two atomic orbitals combine to form molecular orbitals, the wave functions are combined both in-phase and out of phase to create one bonding molecular orbitals and one anti-bonding molecular orbitals respectively.
Let two atoms A and B combine to form a molecule AB.
A+B→AB
i.e. Ψmo= ΨA±ΨB
where ΨA=wave function of atomic orbital of atom A,
ΨB=wave function of atomic orbital of atom B
Ψmo =wave function of molecular orbital of AB
Here, Ψb=ΨA+ΨB and Ψ*=ΨA-ΨB can be separated from Ψmo= ΨA±ΨB.
Equation Ψb=ΨA+ΨB represent the formation of bonding molecular orbital formed by the addition overlap of atomic orbitals.
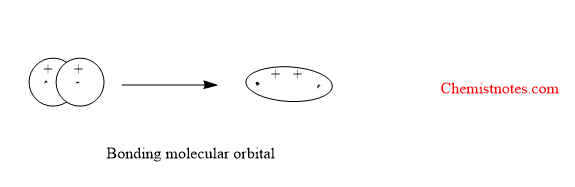
Equation Ψ*=ΨA-ΨB represents the formation of anti-bonding molecular orbital formed by subtraction overlap of atomic orbitals.
Conditions of LCAO method
The main conditions of the LCAO method are:
- The atomic orbitals must have comparable energy.
- The atomic orbitals must overlap appreciably
- The atomic orbitals must have same symmetry with respect to the molecular axis.
Bond order molecular orbital theory
The bond order is defined as half of the difference between the number of bonding electrons(Nb) and antibonding electrons(Na).
Mathematically, Bond order= (Nb-Na)/2
- Bond order of a diatomic molecule is equal to number of bonds present between two atoms. For examples: bond oder of O2 molecule= 2
- Zero bond order of a moelcule represent that it is unstable and does not exit.
- Bond order is directly proportional to stability of molecules, bond energy but inversly proportional to bond length of molecules.
How to find bond order in molecular orbital theory
For O2 molecule:
The electronic configuration of O-atom= 1s2, 2s2, 2p4
Total electrons in O2 molecules= 16
Molecular electronic configuration of O2 molecules= σ1s2, σ*1s2, σ2s2, σ*2s2, σ2px2, π2py2=π2pz2, π*2py1=π*2pz1
Bond order= (Nb-Na)/2=(10-6)/2= 2
Bond order 2 means there is a double bond in O2 molecules.
Valence bond theory and molecular orbital theory differences
Atomic orbitals and molecular orbitals similarities
Some similarities between molecular orbitals and atomic orbitals are given below:
- Electron whether it is present in atomic orbitals or molecular orbitals, can be denoted by a particular wave function, Ψ.
- Like atomic orbitals, molecular orbitals also accommodate maximum two electrons with oppostie spin.
- Like atomic orbitals, the molecular orbitals also have different shapes and energy.
- Electrons should follow the aufbau principle,pauli’s exclusion principle and hund’s rule when it enters in Atomic orbitals or molecular orbitals.
Atomic orbitals and molecular orbitals differences
The main differences between atomic orbitals and molecular orbitals are:
| Atomic orbital | Molecular orbital |
| In atomic orbitals, an electron is influenced by only one nucleus. | In molecular orbitals, an electron is influenced by two or more nuclei. |
| They have simple shapes | They have complex shapes |
| Atomic orbitals are an inherent property of an atom. | Molecular orbitals are formed by the mixing of atomic orbitals. |
How to write electronic configuration in molecular orbital theory
The electrons are filled in molecular orbitals in the case of a molecule. The electrons may occupy either bonding molecular orbital or antibonding molecular orbitals. In some molecules, electrons may occupy non-bonding orbitals as well. The bonding orbitals are represented by σ and π while antibonding orbitals are represented by σ* and π*. The electronic configuration in molecular orbitals are written as:
For lighter elements(from H2 to N2)
The relative energy of molecular orbitals is
σ1s<σ*1s<σ2s<σ*2s<π2py=π2pz<σ2px<π*2py=π*2pz<σ*2px
For heavier elements(O2,F2 and Ne2)
The relative energy of molecular orbitals is
σ1s<σ*1s<σ2s<σ*2s<σ2px<π2py=π2pz<π*2py=π*2pz<σ*2px
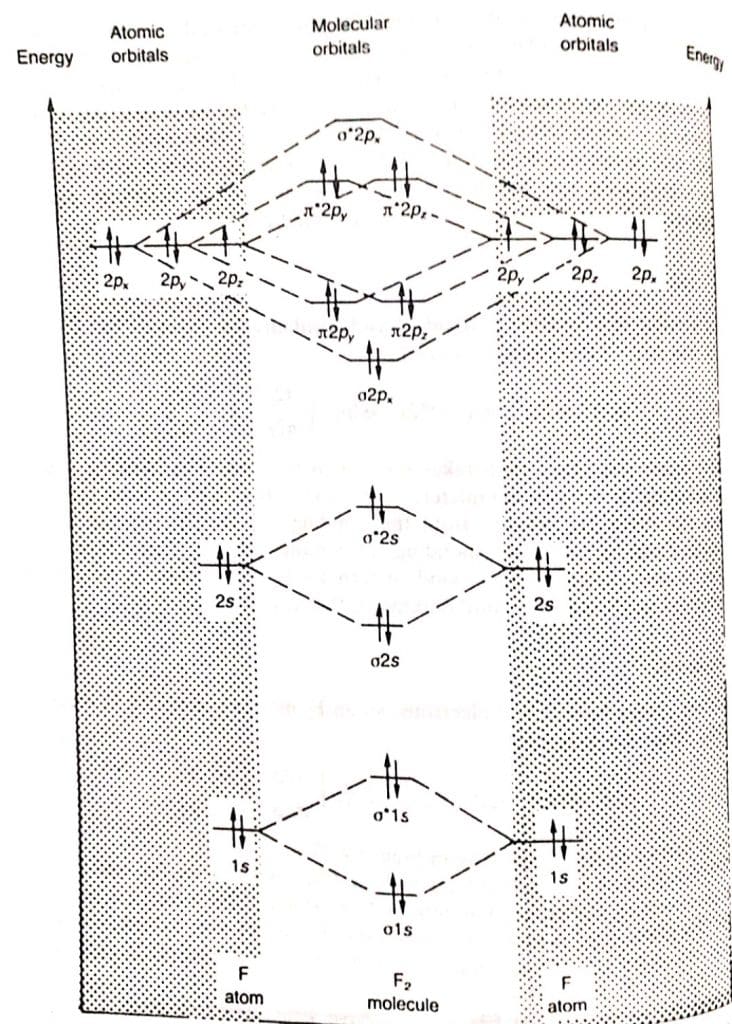
Molecular orbital theory diagram
The molecular orbital diagram of flourine as example is shown below:
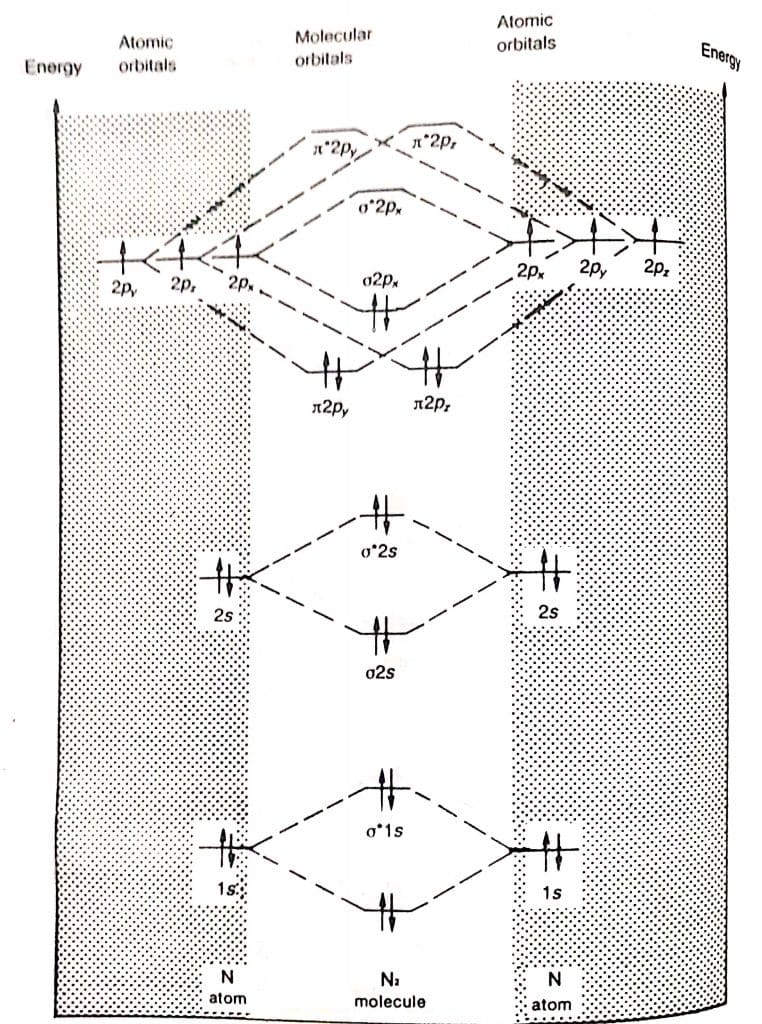
formation of N2 molecule by molecular orbital theory
Each nitrogen atom has 7 electrons. Thus, the N2 molecule contains a total of 14 electrons. These electrons are arranged in molecular orbitals as:
σ1s2, σ*1s2, σ2s2, σ*2s2, π2py2=π2pz2, σ2px2, π*2py0=π*2pz0
In such molecules, the energies of π2py=π2pz orbitals are lower than σ2px orbitals.
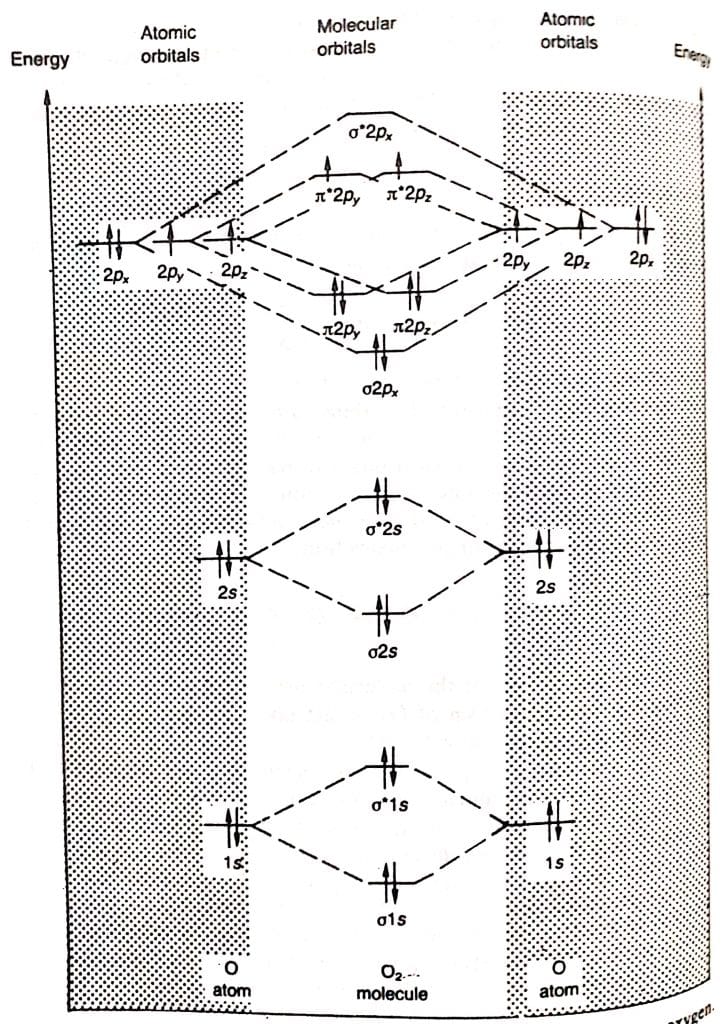
molecular orbital theory of O2
Each oxygen atom has 8 electrons. Thus, the O2 molecule contains a total of 16 electrons. These electrons are arranged in molecular orbitals as:
σ1s2, σ*1s2, σ2s2, σ*2s2, σ2px2, π2py2=π2pz2, π*2py1=π*2pz1
The antibonding orbitals π*2py, π*2pz are singly occupied in accordance with Hund’s rule.
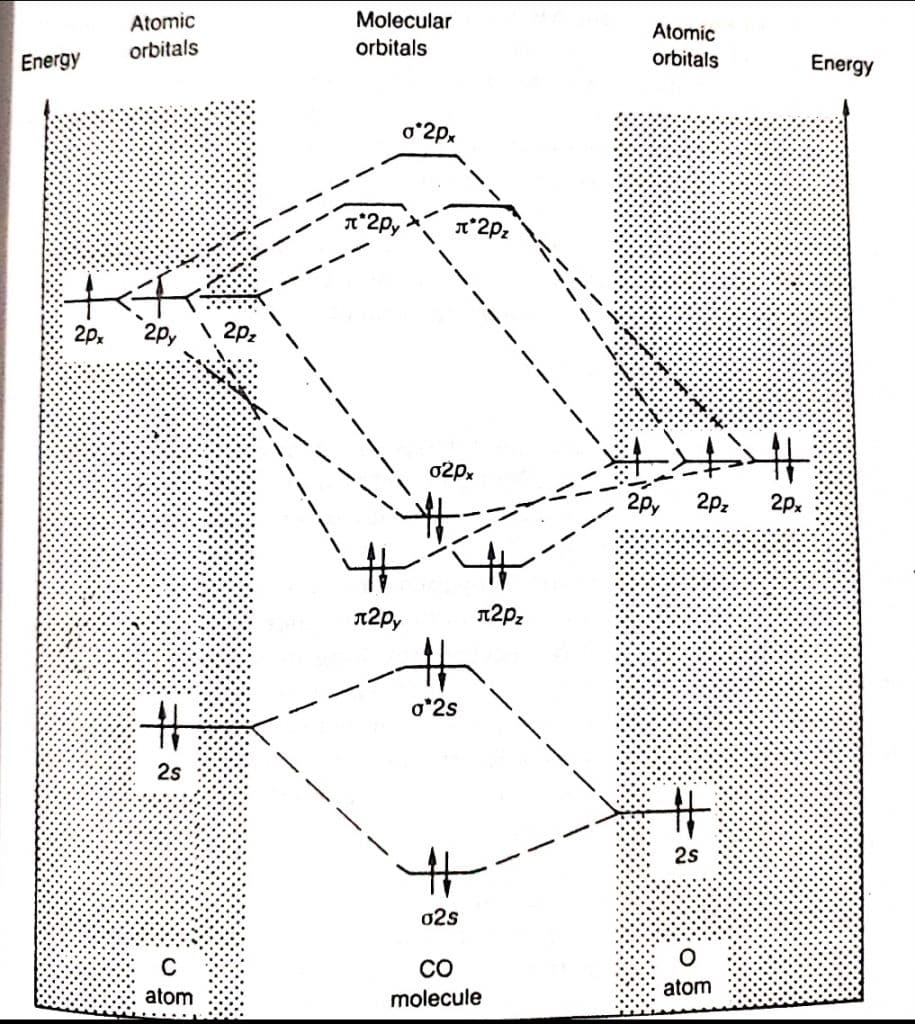
Molecular orbital theory of CO
The molecular orbital theory diagram of the CO molecule is shown below: