Table of Contents
ToggleAufbau principle states that an electron occupies an orbital in the order of lowest to highest energy orbital. ‘Aufbau’ is a german word that means construction or build up. This is the reason, it is also called a building-up principle or construction principle. Basically, this principle explains how electrons are distributed among energy levels in the ground states of atoms. The electrons are filled in an orbital following Pauli’s exclusion principle as well as Hund’s rule.
Aufbau Principle Definition
According to the Aufbau principle, “electrons are filled in an orbital in the increasing order of orbital energy level“. In other words, electrons are filled in the orbital in such a way that the orbital with the lowest energy gets filled first, then to the next higher energy level, and so on i.e. in the pattern of their increasing energy.
The correct sequence of filling of electrons in an orbital depends on two factors.
- Orbital having the lowest value of (n+l) is filled first
- In the case where two orbitals have the same (n+l) value, the orbital with the lowest ‘n’ value will be filled first by electrons.
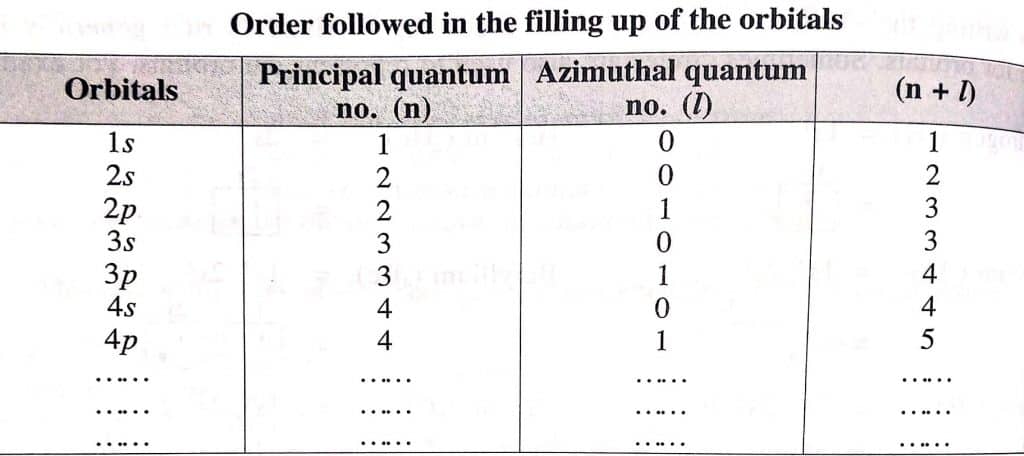
Aufbau Principle diagram
The diagram given below explains the correct sequence of filling up of energy levels.
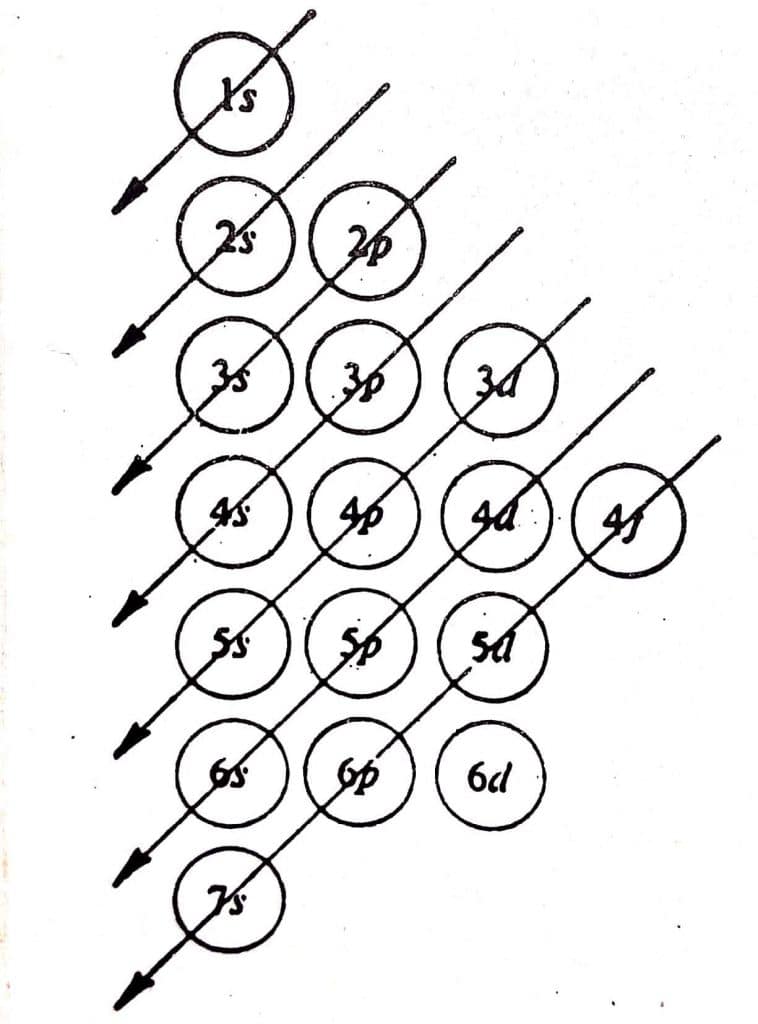
As shown in the figure, electrons will be first filled in 1s orbital as it possesses the lowest (n+l) value. After filling of 1s orbital, electrons will be consequently filled in 2s, 2p, 3s, and so on. In the case of 2p and 3s orbitals, both have same (n+l) value i.e. (2+1) for 2p and (3+0) for 3s. Thus the factor 2 explained above operates and the orbital with the lowest ‘n’ value gets filled first. This is the reason, 2p orbital is filled first before 3s orbital.
Aufbau Principle Example
Let us write the electronic configuration of Chlorine (Cl).
Chlorine has an atomic number of 17, which means that it possesses 17 electrons. According to this Principle, as well as Hund’s rule and Pauli exclusion principle, the first two-electron will occupy in 1s orbital as this orbital possesses the lowest (n+l) value of 1. Then electron will occupy at 2s orbital having (n+l) value of 2, and then to the 2p orbital which has 3 ( (n+l) value. 3s orbitals are filled after 2p orbital though both have the same (n+l) value of 3. In such cases, the orbital with a low ‘n’ value is filled first, and hence 3s orbital is filled after 2p orbital.
Then, the remaining five electrons occupy at 3p orbitals which have (n+l) value of 4. Thus, the electrons are filled in the increasing order of energy levels. Now, the electronic configuration of Chlorine according to the Principle is 1s22s23p64s23p5
Aufbau Principle Violation/Exception/Limitations
Some of the elements show quite much deviation from this principle. This deviation/exception is due to the shifting of an electron from its ns orbital to (n-1)d orbital to achieve fully-filled or half-filled orbitals. The fully-filled or half-filled orbitals possess greater stability.
One of the common elements that show exceptions to this principle is Chromium and Copper.
In the case of Chromium, the expected electronic configuration is [Ar]3d44s2. But the correct electronic configuration of chromium that shows deviation from the principle us [Ar]3d54s1.
As we know that half-filled and full-filled orbitals possess greater stability, the expected electronic configuration does not possess half-filled or full-filled orbital, while the correct configuration on shifting its one 4s electron to 3d electrons ( [Ar]3d54s1 ) acquires half-filled orbital, and is, therefore, the stable configuration than [Ar]3d44s2.
In the context of copper, the correct electronic configuration is [Ar]3d104s1 rather than [Ar]3d94s2.This is due to the reason that Cu shifts its one 4s electron to 3d so that the 3d orbitals get fully filled.
As we know that half-filled and full-filled orbitals possess greater stability, the electronic configuration [Ar]3d104s1 is more stable, and therefore correct than the expected electronic configuration of copper [Ar]3d94s2.
Application of Aufbau Principle
- It explains the manner in which the electrons are filled in the orbital.
- It is used to determine the electronic configuration of an atom or ion.
Hund’s rule, Aufbau Principle, Pauli Exclusion Principle
Hund’s rule states that” In the orbitals of the same subshell, electrons are filled singly first before pairing starts.”
According to the Aufbau principle, “electrons are filled in an orbital in the increasing order of orbital energy level“.
The Pauli Exclusion principle states that no two electrons in an atom or molecule can have the same quantum numbers.
Aufbau Principle Video
FAQs/MCQs
Explain Aufbau Principle with an example
The Aufbau principle states that electron occupies orbital in the order of lowest to highest energy orbital. Let us write the electronic configuration of Chlorine (Cl). Now, the electronic configuration of Chlorine according to the Aufbau Principle is 1s22s23p64s23p5






