Table of Contents
ToggleWerner’s Theory of coordination compounds is a hypothetical concept to explain the formation of complex compounds. This theory was given by Alfred Werner in 1983. He was awarded the Nobel Prize in chemistry in 1913 for his work on coordination compounds.
Postulates of werner’s theory of coordination compounds
Werner is also known as the father of Coordination chemistry. He proposed some theoretical concepts to explain how the coordination compounds are formed. The major postulates of Werner’s Coordination theory are given below:
- The central metal atom of the coordination compounds shows two types of valencies. .i.e., primary valency and secondary valency.
- The primary valency is also known as principle valency which is ionizable. But secondary valency is known as auxillary valency which is not ionizable.
- The primary valency of the central metal atom represents the ionization states whereas the secondary valency represents the coordination number. Co-coordination number is defind as the total number of ligands attached directly with the central metal atom or ion in a complex.
- Every metal atom has a fixed number of secondary valencies i.e., it has a fixed number of coordination number.
- The primary valency of the central metal atom is satisfied by negative ions, but secondary valencies may be satisfied by negative ions or neutral molecules.
- The secondary valencies of the metal are always directed towards fixed position in space and this leads to definite geometry of the coordination complex. It means the primary valencies are not directional in nature but the secondary valencies are directional in nature.
For example: If the metal ion has four secondary valencies then these are oriented in either tetrahedral or square planar geometry around the central metal atom. Therefore, secondary valency is responsible for the determination of stereochemistry or geometry of the complex.
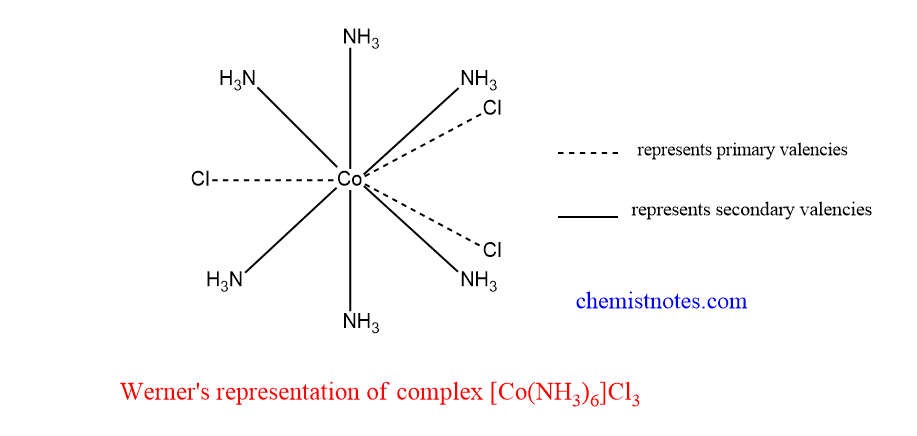
Werner’s representation of complex
Let’s take the example of CoCl3.6NH3 Complex.
When this complex reacts with AgNO3 solution, it gives white precipitate. This means it has an ionizable Cl- ion. Qualitative analysis showed that one molecule of this complex CoCl3.6NH3 liberated 3 Cl– ions. Therefore, 3 Cl-atoms are bonded to Cobalt-metal atoms by primary valencies.
Similarly, the solution of this complex does not respond to the test for cobalt. From this result, it can be concluded that a cobalt metal atom with 6 NH3 molecules is bound inside the coordination sphere. These 6 NH3 molecules balance the secondary valencies of Cobalt metal and these are directed along the corner of a regular octahedron. Therefore, the formula of the complex is given as [Co(NH3)6]Cl3.
Limitations of werner’s coordination theory
Although Werner’s theory has tried to explain the formation of complex compounds, it has some limitations as well.
- It does not explain why certain elements can form complex compounds while others elements don’t.
- It fails to explain the directional nature of bonds and definite geometry of the complex.
- It does not explain the magnetic and optical properties of the complex.
References:
- F. A. Cotton, G. Wilkinson and P. L. Gaus, Basic Inorganic Chemistry, (6th Edition),
John Wiley and Sons, 1995 - J. Lewis and R.G. Wilkins, Modern Co-ordination Chemistry, Interscience Publishers.






