Table of Contents
ToggleDefinition: Hund’s rule refers to a set of rules that are used as the guidelines for filling electrons in the orbital and spin of an electron in the orbital. German physicist Friedrich Hund formulated a set of rules for filling electrons in an orbital in 1927. Out of his three statements, two are used in chemistry which is used for the placement of electrons in orbital.
According to Hund’s rule:
- Doubly fulfillment of orbital is only possible after singly occupying in the orbital.
- All electrons in a singly occupied orbital have the same spin to maximize total spin
Hund’s rule definition chemistry
Hund gave an empirical rule for the arrangement of electrons in degenerate orbitals, which is termed Hund’s rule. It is also known as Hund’s rule of maximum multiplicity.
Hund’s rule states that”In the orbitals of the same subshell, electrons are filled singly first before pairing starts.”Alternatively, Hund’s rule states that electrons don’t pair up in a subshell until they are occupied by a single spin.
Example of hund’s rule
Let us take an example of filling electrons in a nitrogen atom. The electronic configuration of nitrogen is 1s2 2s2 2p3.
There are two possibilities of filling the electrons in 2p orbital as shown below.
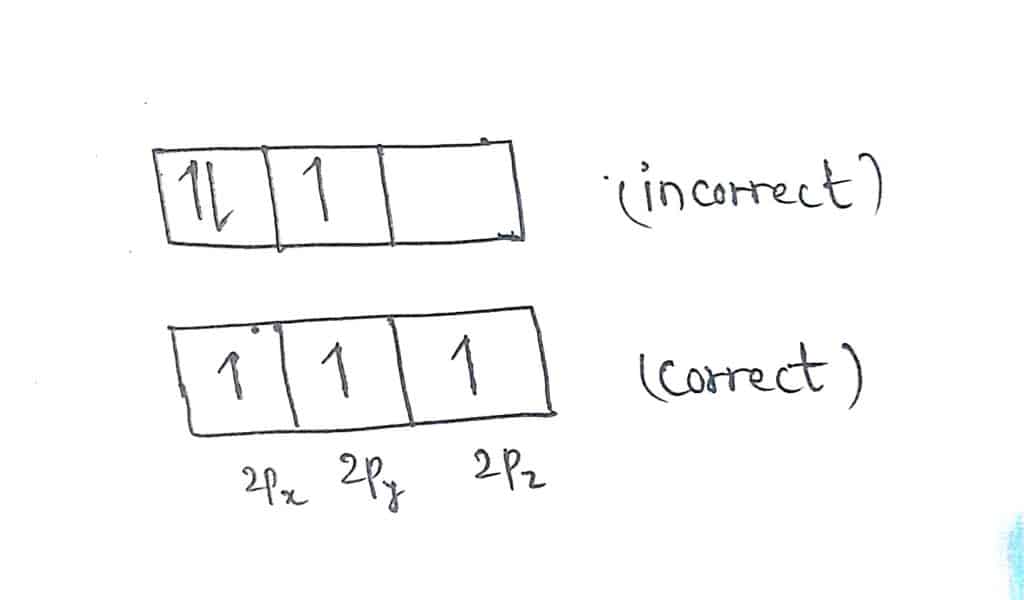
Why hund’s rule is called rule of maximum multiplicity?
According to Hund’s rule, while filling up electrons in degenerate orbitals(orbitals having the same energy level), electrons first fill up singly then only start to pair up. It means atomic orbitals tend to have more unpaired electrons as far as possible. A greater number of unpaired electrons offer maximum multiplicity. Therefore, Hund’s rule is called a rule of maximum multiplicity.
Violation of hund’s rule example
If there is a doubly occupied orbital before singly filled, such arrangement of electrons violates Hund’s rule. Some examples shown below illustrate the condition of violation of Hund’s rule.
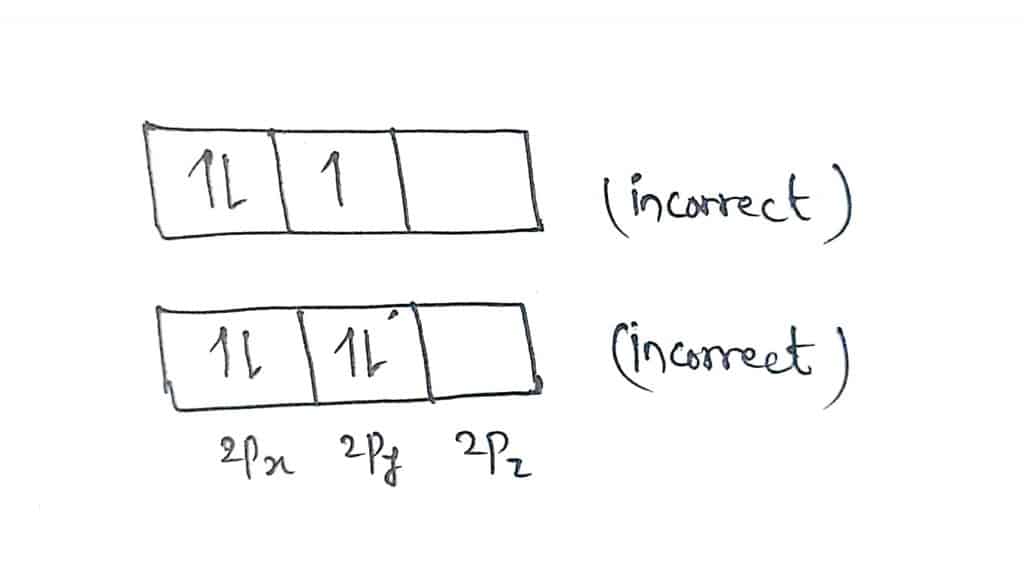
In the first figure, the 2Px orbital contains two electrons before 2Py and 2Pz orbitals are filled singly. Similarly, in the second figure, 2Pz orbital is empty while 2Px and 2Py orbital are filled with two electrons. Such arrangements of electrons in orbitals violate Hund’s rule.
Difference between hund’s rule and aufbau principle
The difference between Hund’s rule and the Aufbau principle is given below:
| Hund’s rule | Aufbau principle |
| Hund’s rule explains that electrons don’t pair up in a subshell until they are occupied by a single spin. | Aufbau’s principle explains that electrons first fill in the lowest energy subshell before filling up in the higher energy subshell of the atom. |
| This rule is governed by maximum multiplicity. | This principle is governed by the (n+l) rule. |
Difference between hund’s rule and pauli exclusion principle
The difference between Hund’s rule and Pauli exclusion principle is given below:
| Hund’s rule | Pauli’s exclusion principle |
| Hund’s rule states that electrons don’t pair up in a subshell until they are occupied by a single spin. | Pauli’s exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. |
Application of hund’s rule
According to Hund’s rule, electrons are singly filled in orbitals then the pairing of electrons takes place. Therefore, the application of Hund’s rule is that it gives us an idea of filling electrons in the atomic orbitals. Similarly, it gives an idea about which arrangement of electrons is correct or more stable.
Limitations of hund’s rule
The limitation of Hund’s rule is that it can not be used to predict the lowest energy configuration.
Hund’s rule video
FAQs/MCQs:
what is Hund’s rule?
Hund gave an empirical rule for the arrangement of electrons in degenerate orbitals, which is termed Hund’s rule. Hund’s rule states that”In the orbitals of the same subshell, electrons are filled singly first before pairing starts.
what does Hund’s rule state?
Hund’s rule states that”In the orbitals of the same subshell, electrons are filled singly first before pairing starts.
what is Hund’s rule in chemistry?
Hund’s rule states that electrons don’t pair up in a subshell until they are occupied by a single spin.
why Hund’s rule is called maximum multiplicity?
Atomic orbitals tend to have more unpaired electrons as far as possible according to Hund’s rule. A greater number of unpaired electrons offer maximum multiplicity. Therefore, Hund’s rule is called a rule of maximum multiplicity.






