Table of Contents
ToggleIn 1911, Ernest Rutherford, a British physicist disputed Thomson’s hypothesis of the atom by demonstrating that atoms as dense, tiny having largely empty space filled with electrons revolving in a fixed path around a positively charged nucleus, like planets revolving around the sun. Rutherford found this by bombarding a thin sheet of gold foil with alpha rays (helium nuclei).
Rutherford’s Nuclear Atomic Model
The nuclear model was named by the physicist name and is known worldwide as Rutherford’s Atomic Model. According to the Rutherford Atomic Model “Protons and neutrons, which constitute almost all of the mass of the nuclear atom, are found in the nucleus and lie in the center of the atom“. The electrons surround the nucleus moving in a circular path with high speed and take up the majority of the atom’s volume”. He proved his statement with an experiment by bombarding a thin sheet of gold foil with alpha rays.
Summarize Rutherford’s model of the atom
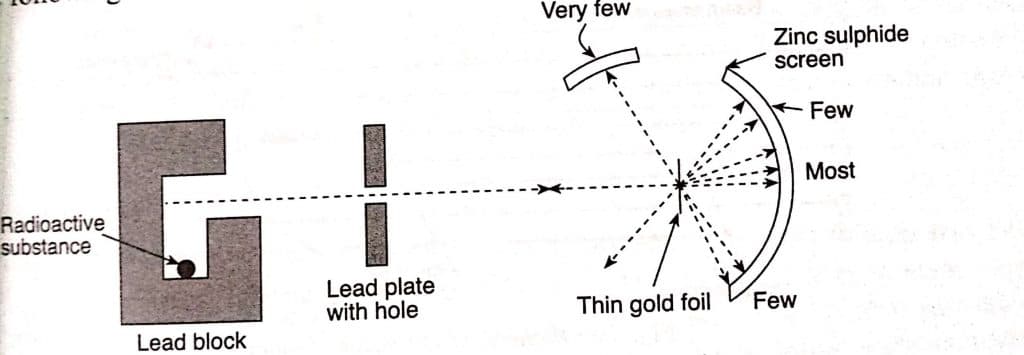
In this experiment, the α-particles radium source is placed inside the lead block, the beam of α -particles (rays) is narrowed by applying a lead slit in the route, and highly energized a-particles are permitted to strike on the thin gold foil equipped with a circular fluorescent zinc sulphide screen as shown in the figure. When a-particles collide with the sulphide screen, they emit light flashes.
The following observations were made during the experiment
- Most of the a-particles (99%) directly passed through gold foil without any deviation.
- Few a-particles deviated from a small angle.
- Only a few a-particles are deflected with angle greater than 90° or rebounded in the back direction.
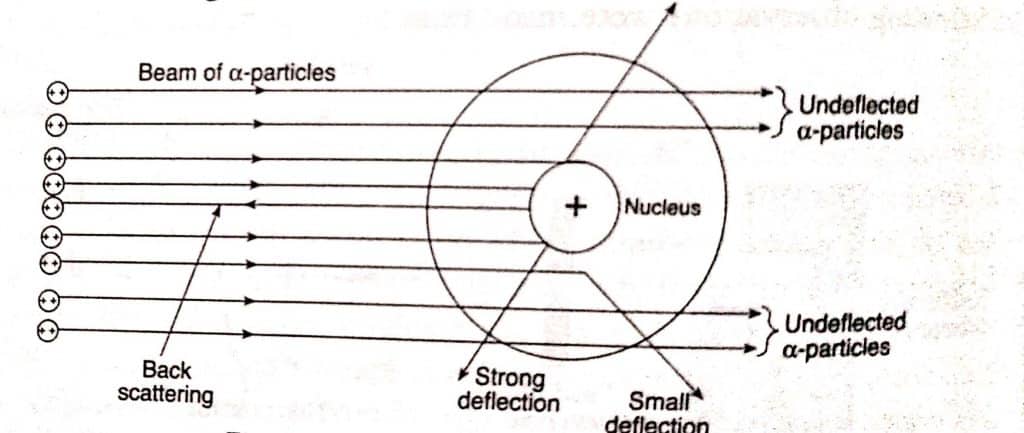
α -particle is a helium nucleus (He) having four unit mass and two-unit positive charge, a-particle will deviate or deflect only when it goes near or strike with another heavier positively charge mass. Based on the above observation, the following conclusions are drawn:
- Since the majority of the alpha-particles traveled straight through gold foil with no deviation. This demonstrates that the atom has a considerable amount of empty space.
- Few a-particles deviate at a modest angle, implying that they must approach a hefty positively charged body within the atom. This demonstrates that the nucleus, which is a dense positively charged mass, is located in the core of the atom.
- Since only a few a-particles deflected with an angle more than 90° or renounced, this indicates that alpha-particles have direct interaction with a hefty positively charged nucleus in a relatively restricted location.
Based on the above experiments, Rutherford postulated an atomic model called Rutherford’s atomic model. The postulates are:
- The positively charged nucleus is located at the center of the atom and is extremally small in comparison to the size of an atom.
- The negatively charged electrons revolve around the nucleus like planets revolve around the sun i.e. electrons are in motion, but not stationary as depicted by Thomson’s atomic model.
- The majority of the space in an atom is empty.
Rutherford’s atomic model states that “Atom consists of positively charged nucleus located in a very small region, where entire mass of an atom is concentrated, with electrons revolving around the nucleus in which centrifugal force of the revolving electron is balanced by the electrostatic force of attraction between the electron and the nucleus.”
State the drawbacks of rutherford’s nuclear atomic model
Some of the drawbacks of Rutherford’s atomic model are:
- The electron orbits around the positively charged nucleus, according to the Rutherford atomic model. If this is the case, the electron’s path around the nucleus will spiral, and the electron will eventually fall into the nucleus, causing the atom’s size to collapse. Fortunately, atoms are unbreakable. As a result, it does not explain atom stability.
- It also doesn’t explain about the emission of the atomic spectrum.
Compare Rutherford and Bohr’s model of the atom
| Bohr’s model of the atom | Rutherford’s model of the atom |
| The core nucleus, which comprises protons, contains the majority of the atomic mass, while electrons are distributed in specified energy levels or shells. | The majority of the atom is empty space. A positively charged nucleus is found at the heart of the atom with negatively charged electrons revolving around the nucleus. |
| Only specific frequency waves are emitted by electrons. | Waves of all frequencies are emitted by electrons. |
| The spectrum of electron emission is a line spectrum. | The spectrum of electron emission is continuous. |
| The model explains the relationship between orbital size and energy of the orbital where the smallest orbital has the lowest energy | It does not explain the relationship between orbital size and energy. |
Compare Thomson’s atomic model with Rutherford’s atomic model
| Thomson’s Atomic Model | Rutherford’s Atomic Model |
| It states that the electron is situated in positively charged particles that are spherical in shape | It states atoms consist of a positively charged nucleus with electrons revolving around it. |
| It explains electrons are stationary. | It explains electrons are in motion. |
Rutherford’s atomic model video
FAQs/MCQs
illustrate rutherford’s experiment to explain the model of an atom.
The Rutherford model of the atom states that the atom is made up of an atomic nucleus and electrons that surround it. Rutherford proposed this atomic model using the gold foil experiment which revealed that the center of the atom has a positively charged solid material, whereas the remainder of the atom contains more empty space and the solid positively charged material was assigned as the nucleus.
How did Bohr expand on Rutherford’s model of the atom?
Bohr explains Rutherford’s atomic model by little revision on Rutherford’s theory. He specified that electrons must revolve in orbits of definite size and energy. The energy of an electron is dependent on the size of the orbit; hence smaller orbits provide low energy. Only when an electron moves from one orbit to another can radiation occur.
Which statement best describes rutherford’s model of the atom?
According to the Rutherford Atomic Model “Protons and neutrons, which constitute almost all of the mass of the nuclear atom, are found in the nucleus and lies in the center of the atom”
How is Bohr’s atomic model different from rutherford’s model?
Bohr’s atomic model explains the presence of discrete energy levels, while Rutherford’s atomic model doesn’t explain the presence of discrete energy levels. Similarly, Bohr’s atomic model is based on quantum theory, while Rutherford’s atomic model is based on classical theory.
What did Ernest rutherford’s model of an atom look like?
Ernest rutherford’s model of an atom looks like a miniature solar system where planets revolve around the sun as electrons revolve around the nucleus.
Was rutherford’s model of the atom correct?
No, Rutherford’s atomic model failed to explain the stability of the atom, which was later explained by Bohr’s in his atomic model, Bohr’s atomic model.
Why is rutherford’s model called the nuclear atom?
As Rutherford concludes that the nucleus lies within an atom, Rutherford’s model is popularly called the nuclear model.






