Table of Contents
ToggleWhat are alkali metals?
Alkali metals are s block elements that are located on the left-hand side of the periodic table. Alkali metals are among the most reactive elements on earth as they shed electrons easily. Alkali metals include elements from the IA group such as lithium(Li), sodium(Na), potassium(K), rubidium(Rb), cesium(Cs), and francium(Fr). Since they easily dissolve in water to form hydroxide, which is very alkaline in nature, these metals are known as alkali metals. They truly serve as periodic table representative elements.
Discovery of alkali metals
Johan August Arfwedson, a Swedish scientist, made the initial discovery of lithium in 1817 while examining mineral ore. German chemists Gustav Kirchhoff, who created Kirchhoff’s equations for electrical current, and Robert Bunsen, who gave his name to the Bunsen burner, discovered cesium and rubidium in 1860 and 1861, respectively. Marguerite Perey, a French scientist, made the discovery of francium at the Curie Institute in Paris in 1939. Francium is the most reactive alkali metal we currently know of.
General characteristics of alkali metals
Electronic configuration
In their outermost shell s- orbital, they only have one electron. Therefore, ns1, where n stands for the Valence shell, can be used to describe the overall electronic configuration of the outermost shell.
| Elements | Symbol | Atomic no. | Configuration |
| Lithium | Li | 3 | (He) 2s1 |
| Sodium | Na | 11 | (Ne) 3s1 |
| Potassium | K | 19 | (Ar) 4s1 |
| Rubidium | Rb | 37 | (Kr) 5s1 |
| Cesium | Cs | 55 | (Xe) 6s1 |
| Francium | Fr | 87 | (Rn) 7s1 |
Physical properties of alkali metals
Alkali metals have low metallic attractions as a result of their larger size and single Valence electron, which makes them soft solids. When freshly cut, they have a brilliant luster, but moist air causes them to tarnish soon.
Ionization energy
Being relatively bigger in size, alkali metals show low ionization energy. It means that they lose the first electron easily. The ionization energy of alkali metals decreases downward in the group.
| Elements | Li | Na | K | Rb | Cs |
| I.E ( KJ/ mole) | 520.3 | 495.8 | 418.9 | 403.0 | 375.7 |
Size of atoms and ions
Because they exhibit the least attraction between the nucleus and outermost electrons, alkali metals have the biggest atomic and ionic sizes. From Li to Fr, their atomic sizes and ions increase.
| Elements | Li | Na | K | Rb | Cs |
| Atomic radium A° | 1.23 | 1.54 | 2.03 | 2.16 | 2.35 |
| Ionic radium A° | 0.60 | 0.95 | 1.33 | 1.48 | 1.69 |
Melting and boiling point
Due to their larger size and single valence electron, alkali metals exhibit poor metallic bonding. As a result, their melting and boiling points are low.
| Elements | Li | Na | K | Rb | Cs |
| Melting points | 180.5 | 97.8 | 63.7 | 38.9 | 28.7 |
| boiling points | 1330 | 992 | 760 | 688 | 670 |
Oxidation state
Only one electron is present in the Valence shell of alkali metals. Alkali metal will acquire a stable configuration to a noble gas if this electron is removed. Hence, they exhibit a +1 oxidation state.
Electropositive or metallic character
Alkali metals have low ionization energy and can lose electrons easily. Therefore, they are said to have an electropositive or metallic character. This is the reason why the electropositive or metallic character of alkali metals increases down the group.
Coloration to the flame
Alkali metals’ valence electrons are merely tenuously bound to the nucleus. The electrons in the higher energy level will therefore be able to jump due to the energy produced by heating. They return by emitting absorbed energy in the form of light in the visible region, but they are unstable at higher energies. Consequently, the atom gives the flame its color. The amount of energy absorbed will determine this. As a result, different atoms have various hues.
| Elements | Li | Na | K | Rb | Cs |
| Colour | crimson | golden yellow | pale violet | violet | violet |
| Wavelength( nm) | 671 | 589 | 767 | 780 | 456 |
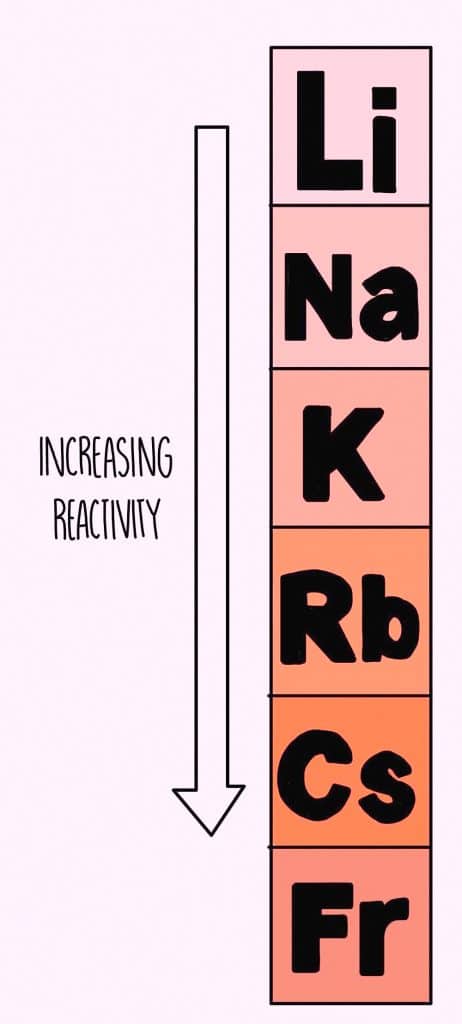
Reactivity
Alkali metals are highly reactive in nature because of their greater tendency to lose electrons. The reactivity increases from Li to Cs.
Why alkali metals cannot be obtained by the chemical reduction method?
Alkali metals have a very low reduction potential and are highly electropositive. Because of this, these metals are strong reducing agents and are easily oxidized. Alkali metals are therefore exceedingly challenging to decrease. Alkali metals, on the other hand, have a stronger affinity for oxygen than other elements do. They also create their carbides when heated vigorously with carbon to decrease. As a result, the chemical reduction process is ineffective for reducing alkali metals.
Reason behind the softness of alkali metals?
The atoms of alkali metals have a large size. They have one electron in their outermost energy level. They form a weak metallic bond because of low attraction between Kernels and mobile electrons.
Chemical properties of alkali metals:
Reactivity of alkali metals towards oxygen :
In oxygen, alkali metals burn vigorously and produce oxides. In contrast to sodium, lithium produces monoxide. Superoxide is formed by other elements. Only when massive cations like K, Rb, and Cs are present does the superoxide O2– ion remains stable.
Na + O² gives Na²O
| Alkali metals | oxide | Peroxide | Superoxide |
| Lithium | Li2O | Li2O2 | |
| sodium | Na2O | Na2O2 | |
| potassium | KO2 | ||
| rubidium | RbO2 | ||
| Cesium | CsO2 |
Reactivity of alkali metals towards air and moisture:
On exposure to the environment, all alkali metals convert into oxide, hydroxide, and then carbonates.
Reactivity of alkali metals towards hydrogen:
Generally, when alkali metals react with hydrogen, they form corresponding hydrides.
Na + H2 → NaH (at high temperatures)
NaH + H2O → NaOH + H2
Reactivity of alkali metals towards halogen:
Ionic crystal halides were formed when alkali metals reacted with halogen.
Reactivity towards liquid ammonia:
In liquid ammonia, metals dissolve to produce a highly conductive deep blue solution.
Na + xNH3 → Na+ + e(NH3)x−
Solubility or Hydration energy of alkali metals :
The solubility of alkali metal ions decreases with increasing size, with cesium ions being the least soluble. Lithium is the most soluble. The ionic type and size are related to solubility in water. Smaller ions can be solvated by more water molecules and have a higher charge density. The hydrated ions become more stable as a result and a higher enthalpy of hydration is released.
What happens when an alkali metal reacts with a halogen?
When the alkali metals react with the different halogens (Group 7 of the periodic table), the group of compounds formed are known as the alkali metals halides.
Na + Cl → NaCl
Why are alkali metals so reactive?
Due to their size and low ionization enthalpy, alkali metals are highly reactive metals. Since they only have one electron in their Valence shell and can lose an electron quickly, their reactivity increases as they move down the group.
How many valence electrons do alkali metals have?
Alkali metals have only one electron in their Valence shell (ns¹).
Why is hydrogen grouped with alkali metals?
Since hydrogen also has one electron in its valence shell, it is classified alongside the alkali metals.
Do alkali metals occur freely in nature?
The alkali metals, which are located in group 1 of the periodic table (formerly known as group IA), are extremely reactive metals that do not naturally occur in large quantities. These metals’ outer shells contain just one electron. They are therefore prepared to shed that one electron when they form an ionic bond with other elements.
Why are halogens and alkali metals likely to form ions?
Because they are both just one electron short of having a complete outer shell, halogens and alkali metals are likely to form ions. To be chemically inert, atoms must all have a complete outer shell.
Are alkali metals good conductors of electricity?
Due to their low ionization energy, alkali metals exhibit metallic properties. Because their valence electrons are mobile, they are good electrical conductors.
Why are alkaline earth metals less reactive than alkali metals?
The removal of two valence electrons from an atom requires more energy than the removal of just one. Due to this, alkali metals with a single valence electron are more reactive than alkaline Earth metals, which have two valence electrons.
Uses of alkali metals
- Many things can be made out of pure sodium, including highly effective lamps that utilize sodium vapor.
- A vital role in the biological system is played by potassium. While KOH is used to make soap, KCl is used as a fertilizer.
- Making photoelectric cells uses Cesium.
- While Rubidium and Cesium are very helpful in academic situations but do not have many uses, lithium, sodium, and potassium do.
- Batteries frequently contain lithium, and lithium oxide can aid in the processing of silica.
- Additionally, lithium can be utilized to produce aluminum, air purification, and lubricating greases.
Why should we not touch the alkali metal?
Alkali metals react violently with water, releasing flammable hydrogen gas that may spontaneously catch fire. When it comes into contact with water, it also creates a caustic hydroxide solution or sodium hydroxide. If consumed, breathed, or absorbed via the skin, it can be hazardous.
What is the chemical formula of baking soda?
The chemical formula of baking soda is NaHCO³
What is the chemical formula of washing soda?
The chemical formula of washing soda is Na²CO³.10H2O
What is the chemical formula of caustic soda?
The chemical formula of caustic soda is NaOH
Sodium oxide solution can’t be stored in Zn or Al vessel?
Sodium oxide can’t be stored in these vessels because Zn or Al reacts with sodium to form sodium oxide (aq).
What is the valency of alkali metals?
The valency for alkali metals is one as they contain only one electron in their Valence shell.
Which process is used for the extraction of sodium?
Down’s process is used for the extraction of sodium.






